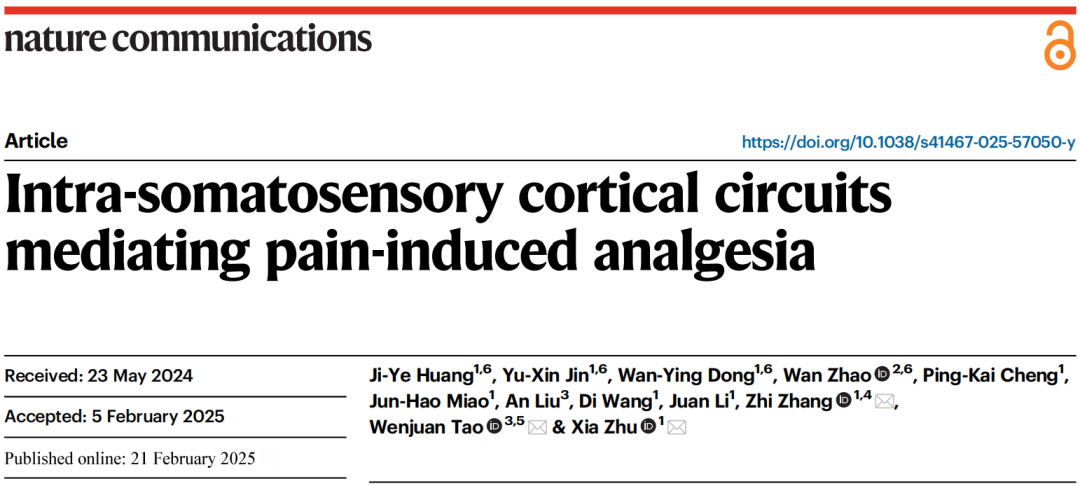
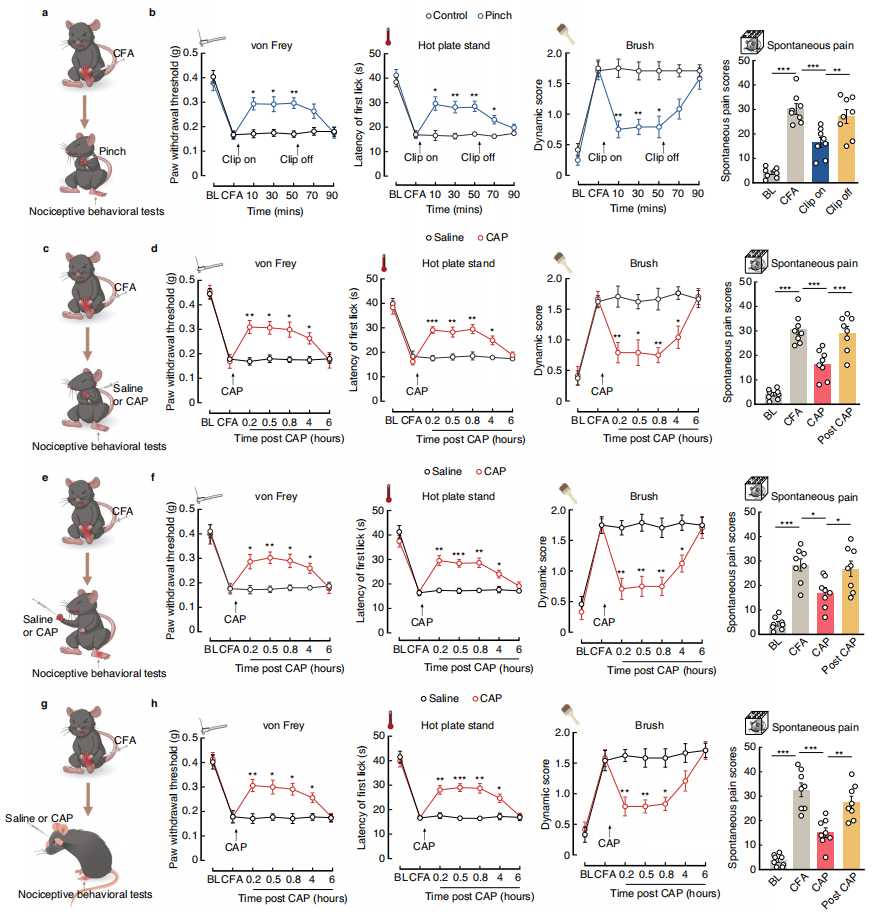
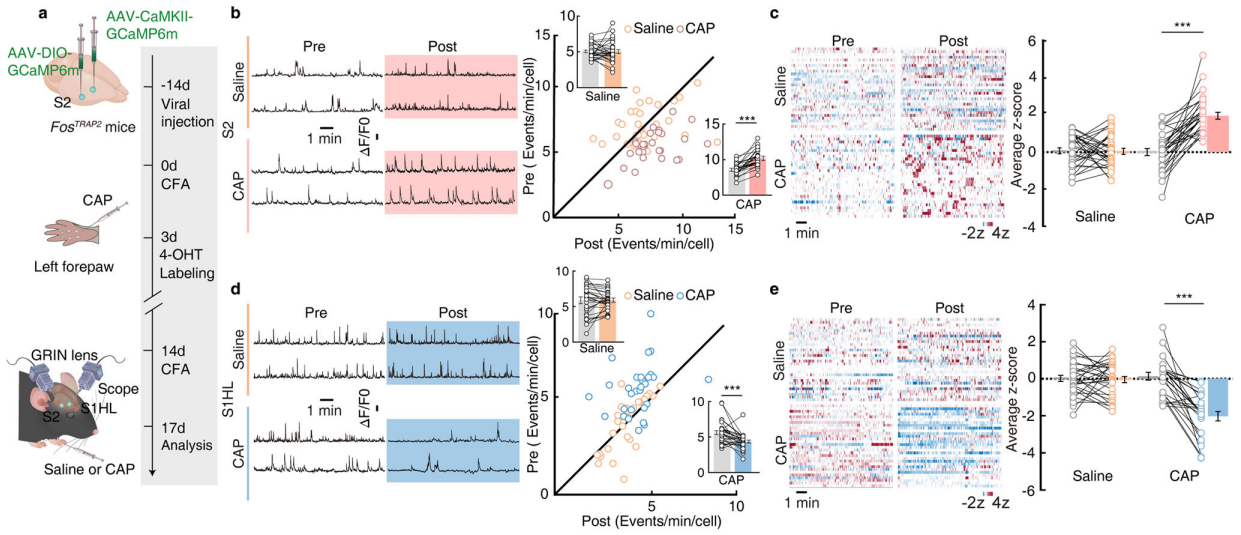
Part 4: S2→S1HL Feedforward Inhibitory Circuit Regulates Analgesia
Using a cell-type-specific anterograde monosynaptic tracing system, researchers injected FosTRAP2 mice’s S2 region with Cre-dependent TK-GFP virus (AAV-DIO-TK-EGFP). Three weeks later, they administered HSV-ΔTK-tdTomato virus (Figure 6a). tdTomato+ signals were detected in multiple regions, including the primary motor cortex, various areas of the primary somatosensory cortex, and the contralateral S2. These signals were primarily distributed in layer 4 of S1HL, with approximately 30% colocalizing with glutamate-specific antibodies and 65% colocalizing with GABA-specific antibodies (Figure 6b-c). This indicates that TRAPed S2 neurons mainly project to GABAergic neurons in layer 4 of S1HL.
To further investigate connectivity, researchers used a cell-type-specific retrograde monosynaptic tracing strategy, injecting Cre-dependent helper viruses into the S1HL region of CaMKII-Cre mice. Three weeks later, they administered rabies virus (RV-EnvA) (Figure 6d). Numerous DsRed-labeled neurons colocalizing with glutamatergic markers were identified in S2. Similar findings were observed in GAD2-Cre mice (Figure 6e-g), confirming that both S1HL-glu and S1HL-gaba neurons receive direct input from S2.
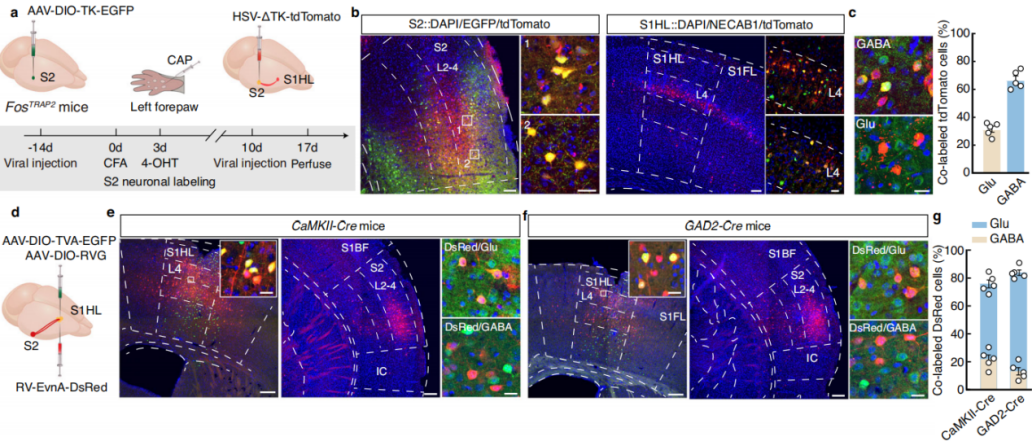 Figure 6: Tracing the S2→S1HL Circuit
Figure 6: Tracing the S2→S1HL Circuit
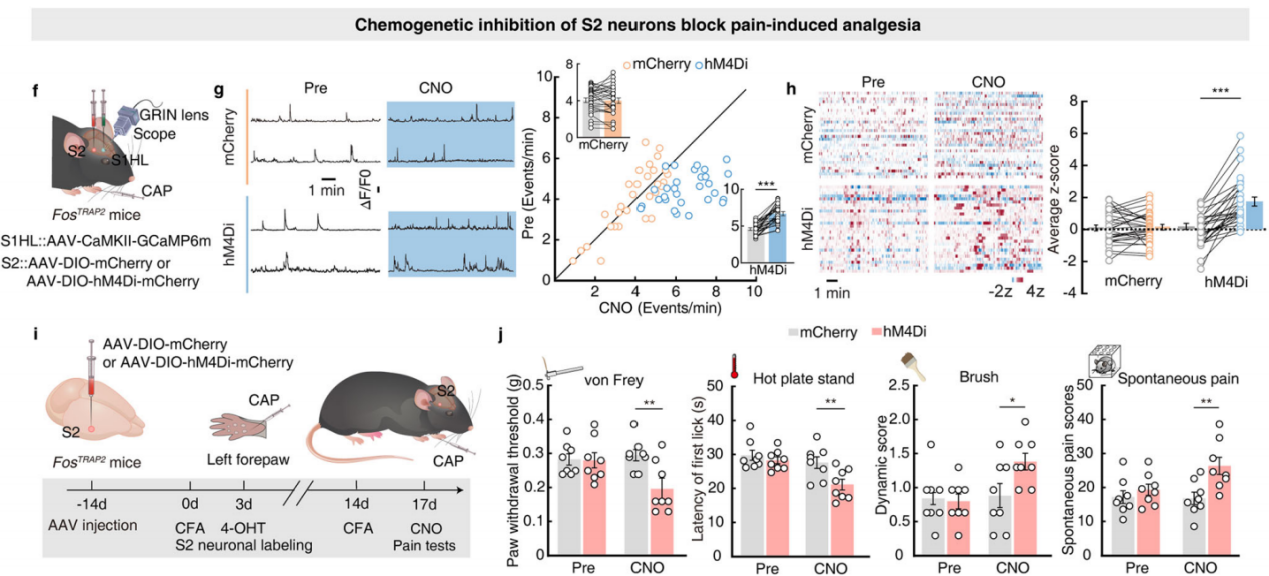
To assess functional connectivity, researchers injected AAV-DIO-ChR2-mCherry into the S2 region of FosTRAP2 mice and AAV-CaMKII-GFP into S1HL (Figure 7h-i), recording optogenetically evoked postsynaptic currents in S1HL neurons. Under a holding potential of -70mV, light stimulation of S2 fibers reliably induced excitatory postsynaptic currents (EPSCs) in S1HL-glu neurons, which were blocked by the AMPA receptor antagonist DNQX. At 0mV holding potential, light stimulation also induced inhibitory postsynaptic currents (IPSCs), which were also DNQX-sensitive, with IPSCs exhibiting a longer latency than EPSCs (Figure 7j-k). These findings suggest that S1HL-glu neurons receive input from local S1HL-gaba interneurons, both of which are directly innervated by S2.
Additionally, after injecting relevant viruses into the S1HL region of GAD2-Cre mice (Figure 7l-m), blue light stimulation of local S1HL-gaba neurons significantly increased IPSCs in S1HL-glu neurons, an effect that was blocked by the GABA receptor antagonist bicuculline (Figure 7n-o). This further confirmed the existence of the S2→S1HL-gaba→S1HL-glu feedforward inhibitory circuit.
Finally, chemogenetic inhibition experiments were conducted by injecting AAV-DIO-hM4Di-mCherry into the S2 region of FosTRAP2 mice and implanting a cannula in the S1HL region (Figure 7p). The results showed that silencing the S2-S1 circuit abolished the increase in hind paw pain threshold, while motor function remained unaffected (Figure 7q). This indicates that noxious stimulation of the left forepaw activates the S2→S1HL-gaba→S1HL-glu feedforward inhibitory circuit, mediating pain-induced analgesia without impairing motor function.
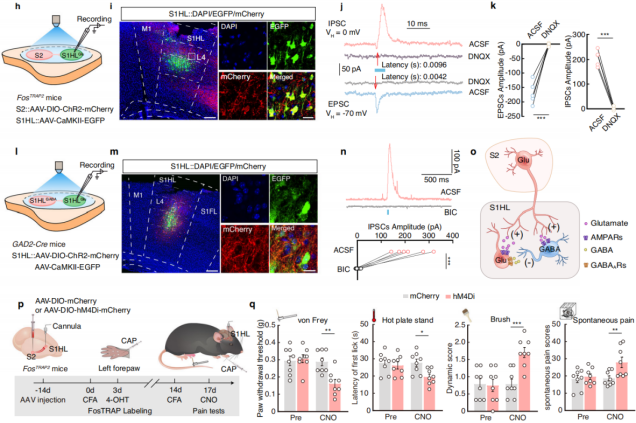 Figure 7: The S2→S1HL-gaba→S1HL-glu Feedforward Inhibitory Circuit Mediates Pain-Induced Analgesia
Figure 7: The S2→S1HL-gaba→S1HL-glu Feedforward Inhibitory Circuit Mediates Pain-Induced Analgesia
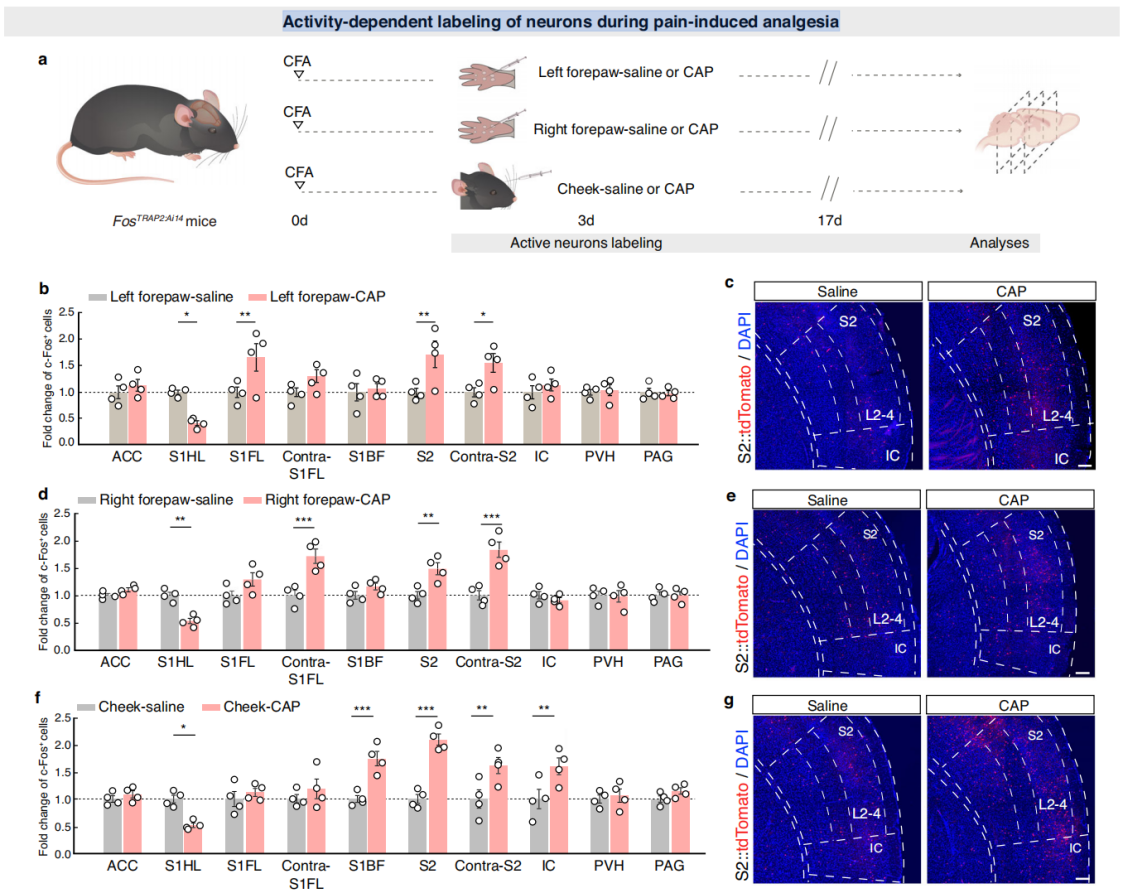
Part 5: Contralateral S2→S2→S1HL Circuit Mediates Contralateral Pain Inhibition
Previous studies have shown that somatosensory processing in mammals is lateralized, meaning sensory information from one side of the body is primarily processed in the contralateral hemisphere of the brain. It is known that harmful stimuli to the right forepaw (on the contralateral body side) can suppress pain-related behavior in the left hindpaw, and the harmful information from the right side of the body is encoded by the contralateral hemisphere. Based on this, researchers hypothesized that contralateral S2 may play a role in mediating the S2→S1HL circuit, possibly contributing to the pain inhibition induced by harmful stimulation of the right forepaw in CFA mice. To test this hypothesis, the researchers employed a three-step retrograde tracing strategy. First, retro-AAV-hSyn-Cre virus was injected into the S1HL of C57 mice, and Cre-dependent helper virus was injected into S2. Three weeks later, RV-ΔG-DsRed virus was injected into S2 (Figure 8a). After the injections, DsRed+ signals were detected in cortical, thalamic, and other brain regions, as well as in contralateral S2 (colocalized with glutamate antibody) (Figures 8b-d). This result suggests that the S2→S1HL circuit indeed receives direct neural input from contralateral S2. The researchers further injected AAV-hSyn-Cre virus into the contralateral S2 of C57 mice and AAV-DIO-YPet virus into S2, using the CLARITY technique to render the brain translucent and enable whole-brain imaging (Figures 8e-f), observing projections of S2 neurons. The results showed significant connectivity between S2 and contralateral S2, as well as projections to the ipsilateral S1 and primary motor cortex (M1) (Figures 8g-h), with numerous subcortical projections, indicating that S2 neurons interact with contralateral S2 and control S1HL.
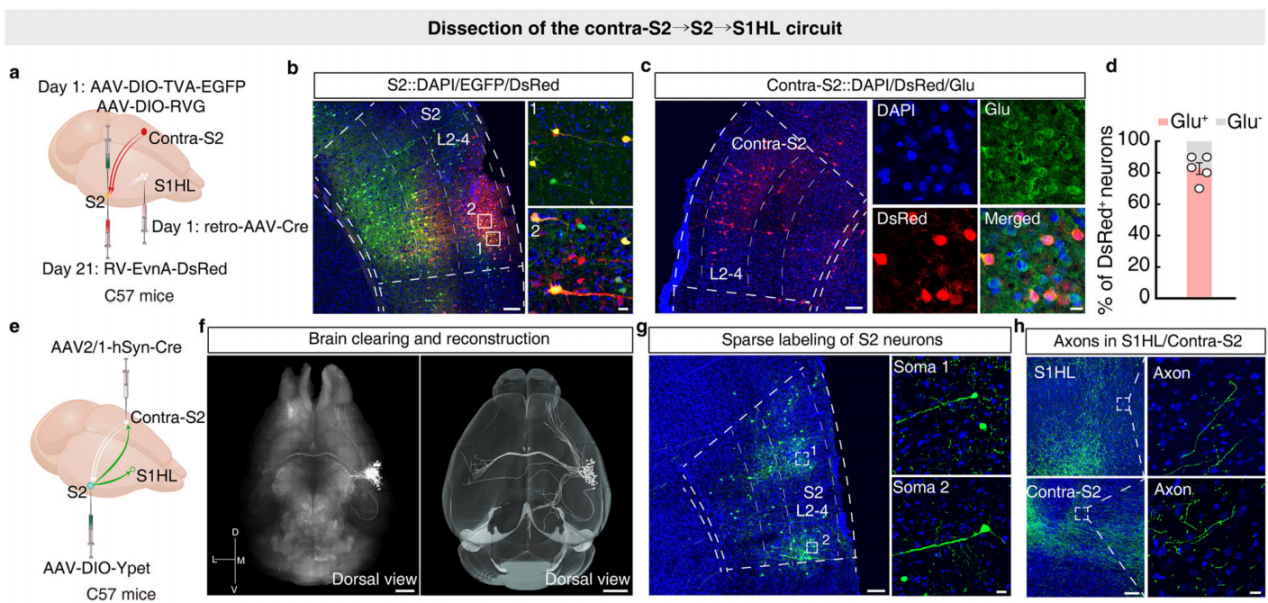
Figure 8: Tracing Contralateral S2→S2→S1HL Circuit
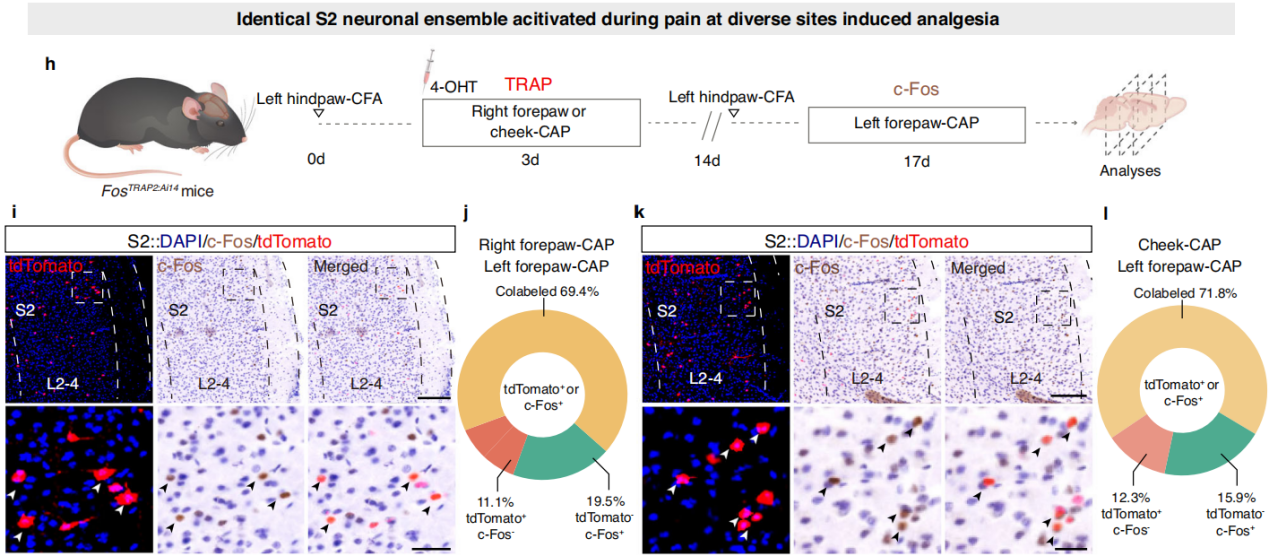
Next, the researchers examined the neuronal activity of contralateral S2, S2, and S1HL-glu during contralateral pain-induced analgesia. They injected AAV-DIO-GCaMP6m and AAV-CaMKII-GCaMP6m viruses into contralateral S2, S2, and S1HL of FosTRAP2 mice (Figure 9i), using microendoscope imaging to monitor calcium responses. After capsaicin injection into the right forepaw of the mice, the transient frequency and average z-score activity of contralateral S2 and S2 neurons significantly increased, while these indicators significantly decreased in S1HL-glu neurons (Figures 9j-o).
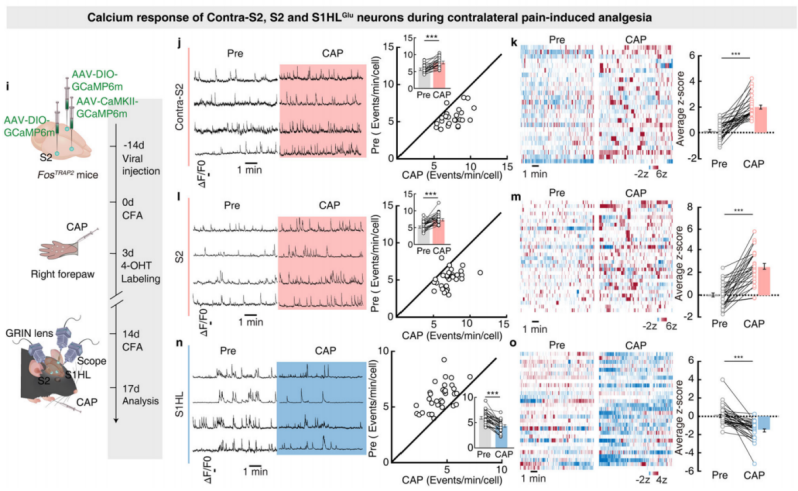
Figure 9: Calcium Activity of Contralateral S2, S2, and S1HL-glu Neurons during Contralateral Pain-Induced Analgesia
Further, chemical genetic viral inhibition of contralateral S2 neurons was performed, and the activity of S2 and S1HL-glu neurons was monitored via AAV-DIO-GCaMP6m and AAV-CaMKII-GCaMP6m viruses (Figure 10a). The results showed that after inhibiting contralateral S2 neurons, the increased activity of S2 neurons and the decreased activity of S1HL-glu neurons in CFA FosTRAP2 mice, induced by capsaicin injection into the right forepaw, were blocked (Figures 10b-e). This indicates that the contralateral S2→S2→S1HL pathway is activated during contralateral pain-induced analgesia. To explore whether the contralateral S2→S2→S1HL pathway is necessary for contralateral pain-induced analgesia, the researchers injected AAV-DIO-hM4Di-mCherry or AAV-DIO-mCherry viruses into contralateral S2 of FosTRAP2 mice and implanted a cannula in S2 for chemogenetic inhibition experiments. The results showed that after inhibition of contralateral S2 neurons, the increase in S2 neuron activity and the decrease in S1HL-glu neuron activity induced by capsaicin injection into the right forepaw were blocked, and the increase in the pain threshold of the hindpaw was also abolished (Figures 10f-g). This confirms that the contralateral S2→S2→S1HL circuit is activated during contralateral pain-induced analgesia and is essential for this process.
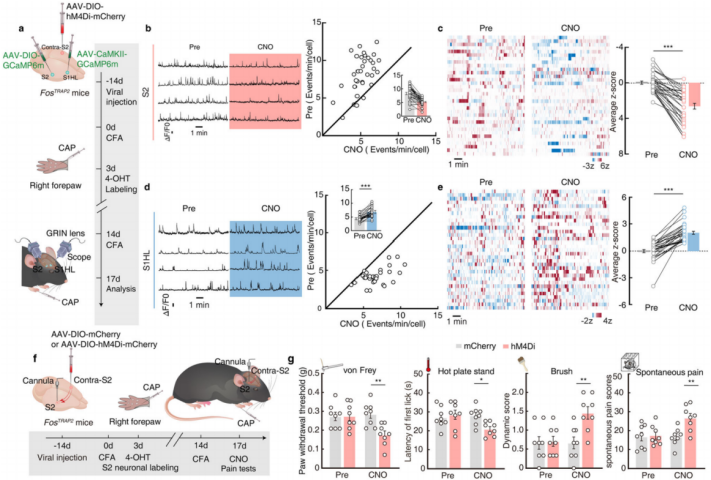
Figure 10: Contralateral S2→S2→S1HL Circuit Mediates Contralateral Pain-Induced Analgesia
Summarize
This study for the first time clearly identified a neural circuit in the somatosensory cortex that mediates "pain inhibiting pain," expanding our understanding of pain relief mechanisms. From a basic research perspective, it reveals the functional heterogeneity of different layers of S2 neurons in pain modulation and emphasizes the importance of the S2-S1 connection in coordinating pain perception across different body parts. From a clinical application standpoint, it provides potential targets for the development of new pain therapies. In the future, precise modulation of these neural circuits may offer more effective ways to alleviate the suffering of chronic pain patients.
The viral tools used in this study are available through Brain Case Biotech: