- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Oncolytic viruses (OVs) replicate and lyse tumor cells in tumor cells through different regulatory mechanisms without affecting normal cell growth [1]. Compared with traditional immunotherapy, oncolytic virus therapy has the advantages of good targeting, less adverse reactions, more ways to kill tumors, and less drug resistance. A number of clinical studies have shown that oncolytic viruses can bring clinical benefits to patients with different types and stages of progression, even metastatic and incurable tumors[2]. More importantly, when it is used in combination with chemotherapy, radiotherapy, immunotherapy, etc., it has a synergistic effect, which can make tumors that have not responded well to immunotherapy drugs such as immune checkpoint inhibitors become sensitive[3].
Today, the oncolytic viruses used for clinical treatment can be roughly divided into the following two categories: one is a naturally occurring virus without gene editing, mainly including reovirus, newcastle disease virus , NDV), natural coxsackie virus A21 (eoxsaekie virus A21, CVA21), etc. The other is genetically modified viruses, mainly including adenovirus, vaccinia virus, herpes simplex virus, vesicular stomatitis virus, etc. [4]. Most OVs are genetically modified to increase the tropism of the virus to tumor cells, improve the selective replication and lytic potential of the virus, and enhance the host's anti-tumor immunity.
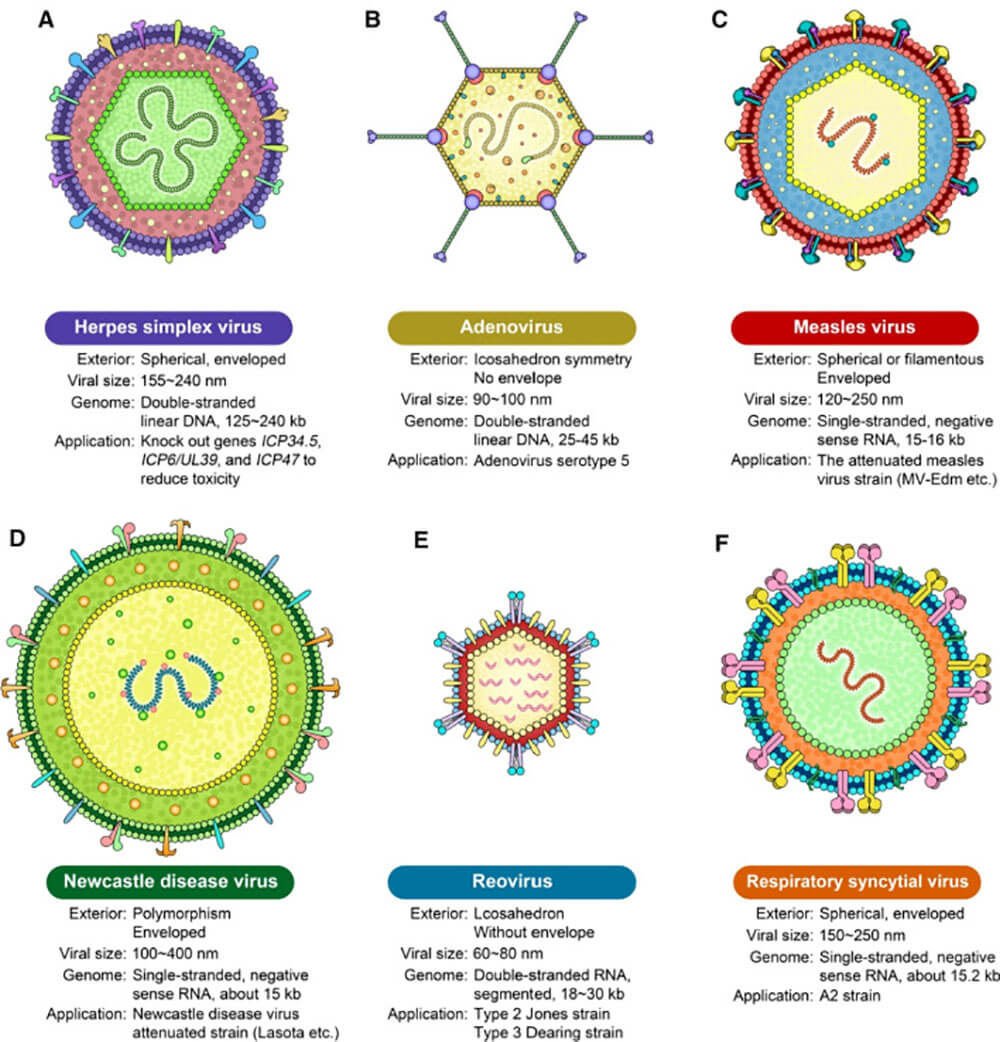
Figure 1: Types of Oncolytic Viruses
According to the definition of oncolytic virus, oncolytic virus selectively replicates in tumor tissue and has no killing effect on normal cells. However, there are many types of oncolytic viruses, and their tumor-specific killing mechanisms are different. Oncolytic viruses selectively infect tumor cells and self-replicate in tumor cells to destroy tumor cells by releasing pathogen-associated molecular pattern molecules (PAMP), tumor-associated antigen (tumor-associated antigen, TAA), inflammation sex factor, substances such as molecules and chemokines activate the body's immune system, directly or indirectly kill tumor cells, and transform cold tumors into hot tumors, as shown in Figure 2.
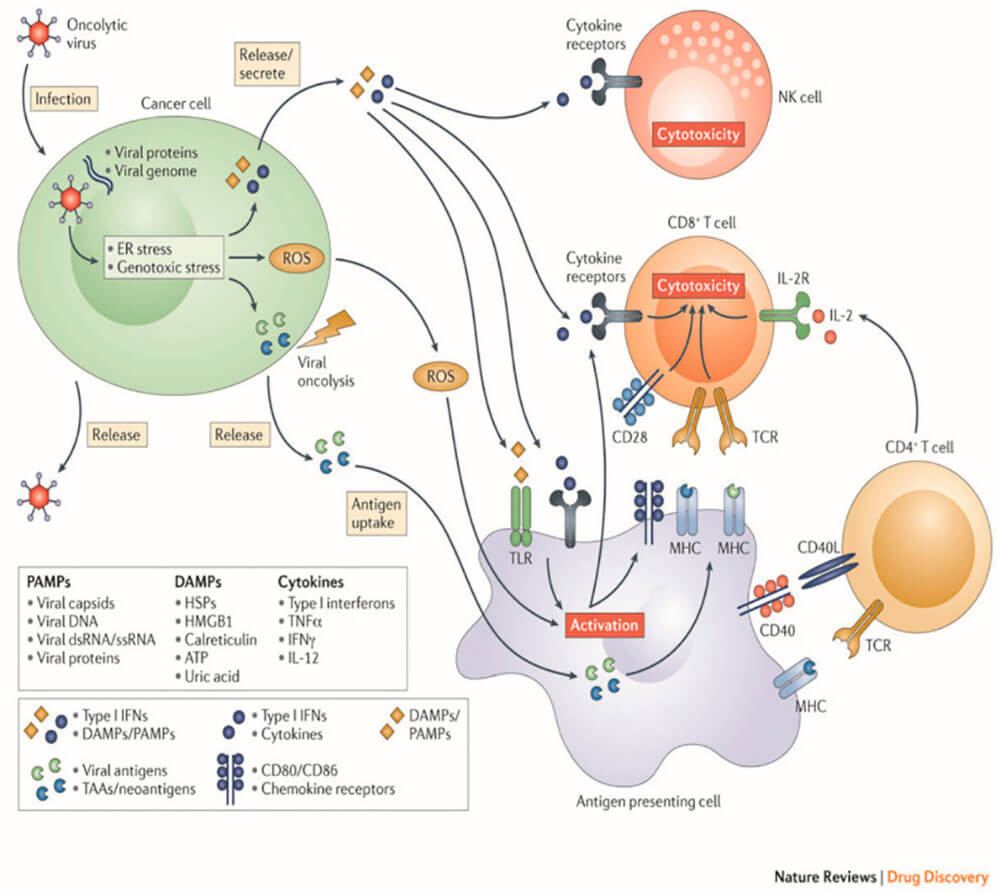
Figure 2: Oncolytic virus mechanism of action
In anti-tumor therapy, there are currently three oncolytic virus drugs approved for clinical use:
Among all clinical trials in 2017, 78 were intervention trials of oncolytic viruses in various malignant solid tumors, which indicated that oncolytic viruses have good prospects for anti-tumor research.
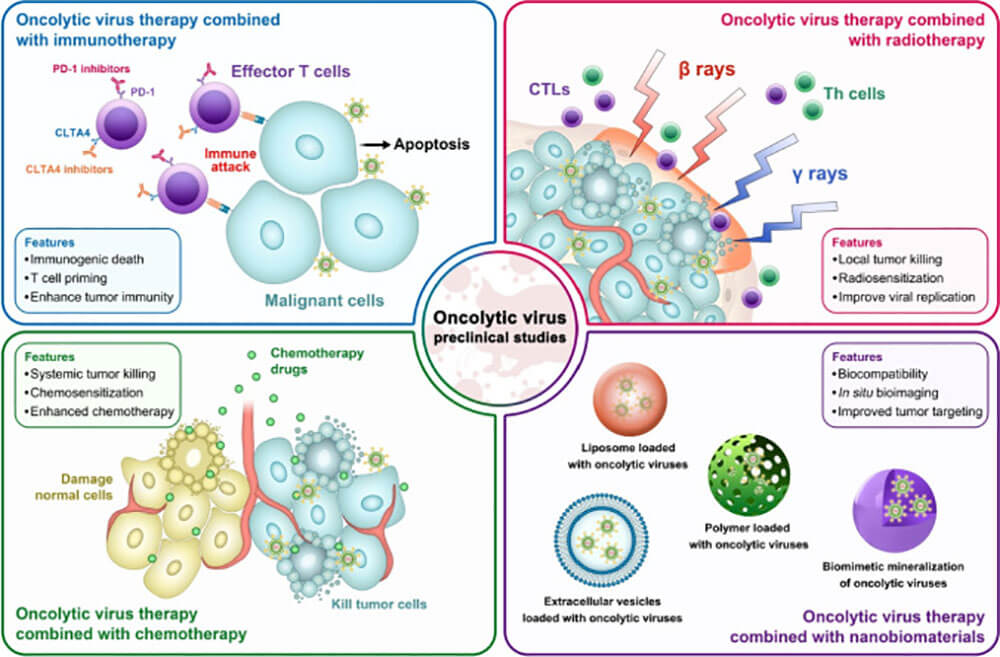
Figure 3: Preclinical features of oncolytic virus combination therapy: combined immunotherapy (top left); combined radiotherapy (top right); combined chemotherapy (bottom left); combined nanobiomaterial therapy (bottom right)
Oncolytic virus is a multifunctional anticancer drug that selectively infects, replicates, and kills tumor cells. This process depends on surface receptors, the tumor cell's permission for virus replication. Oncolytic viruses have shown variable therapeutic efficacy in multiple clinical trials, but rarely induce complete tumor regression in vivo over the long term. Moreover, the selective pressure of heterogeneous tumors results in resistance to oncolytic viruses. In order to overcome these shortcomings, many novel oncolytic virus combination therapies have been established clinically to enhance the lethality of tumors.
Chemotherapy directly kills malignant cells, enhances the immunogenicity of tumor cells, and enhances the cytotoxicity of oncolytic viruses; oncolytic viruses combined with chemotherapy have a synergistic effect and promote anti-tumor immune responses [5]. Mechanisms of combination chemotherapy with oncolytic viruses include:
Targeted therapy works on the same principle as combination chemotherapy, and it also inhibits abnormal signaling pathways in cancer cells[8].
Oncolytic viruses and radiotherapy are two distinct areas of cancer therapy with non-overlapping cytotoxicity profiles. Radiation enhances the oncolytic effect of the virus, and the virus increases the radiosensitivity of cells, and the two have a synergistic effect [5, 9]. Oncolytic virus is a potential tumor treatment strategy. It was initially thought that the cytotoxicity of oncolytic viruses was to increase virus replication, enhance tumor cell infection and oncolysis, but many research data on oncolytic viruses do not support this hypothesis; most of them believed that oncolytic viruses prevent DNA repair and make tumor cells Radiation-sensitizing, induces apoptosis.
Although oncolytic viruses can quickly reduce the size of local tumors, they are not easy to generate a sustained anti-tumor immune response. The immune response is a key component of OV therapy, with strong initial induction followed by suppression of effector cell antitumor activity by various immunomodulators such as CTLA-4 and PD-L1. In a mouse model of malignant melanoma, oncolytic measles virus delivered anti-CTLA-4 and anti-PD-L1 antibodies to the TME, induced a strong specific anti-tumor immune response, and no immune-related toxicity was found[10] . In another mouse model of melanoma, intratumoral injection of NDV combined with anti-CTLA-4 antibody resulted in tumor regression and prolonged survival [12]. PDL1 is produced by overexpression of tumor cells and infiltrating tumor cells, binds to PD-1 on T cells, induces T cell apoptosis [10, 11], and allows tumor cells to escape; while blocking PD-1 improves T cell function. OV infection enhances the expression of immunomodulators, enhances the sensitivity of tumor cells to PD-1 or PD-L1 blockade, and induces anti-tumor immune responses. Therefore, combined immunization with oncolytic virus has therapeutic value.
In recent years, with the rapid development of the field of nanotechnology, the application of bionanomaterials in tumor therapy has received extensive attention [12]. They have good drug loading capacity. Integrating biological imaging, activating tumor immunity and other functions, it can specifically target tumor cells after certain modifications. Both in tumor diagnosis and treatment have shown high potential application prospects. Therefore, biomaterials can be used to load oncolytic viruses to achieve the purpose of blocking viral immunogenicity and achieving tumor-targeted delivery. Currently, liposomes, cells or exosomes are mainly used to carry oncolytic viruses [13, 14], as well as some non-biological material carriers, such as polymers [15].
In the field of drug efficacy and pharmacological evaluation related to nervous system diseases, we can provide you with a one-stop platform for evaluating the behavior of animals from the gene molecular level to the cell tissue level, to the neural circuit, and finally to the animal as a whole.
literature citation
[1] GUJAR S, BELL J, DIALLO J S. SnapShot: cancer immunotherapy with oncolytic uses [J] . Cell, 2019, 176(5):1240-1240. el.
[2] ANDTBACKA R H, KAUFMAN H L, COLLICHIO F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma[J]. J Clin Oncol, 2015,33(25): 2780-2788.
[3] HARRINGTON K J, KONG A, MACH N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study [J] . Clin Cancer Res, 2020, 26(19): 5153-5161.
[4] CAO G D, HE X B, SUN Q, et al. The oncolytic virus in cancer diagnosis and treatment [J] . Front Oncol, 2020, 10: 1786.
[5] Choi AH, O'leary MP, Fong Y, et al. From Benchtop to Bedside: A Review of Oncolytic Virotherapy[J]. Biomedicines, 2016, 4(3):18.
[6] Ko JS, Rayman P, Ireland J, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained[J]. Cancer Res, 2010, 70(9):3526-3536.
[7] Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity[J]. Cancer Res, 2010,70(8):3052-3061.
[8] Roulstone V, Pedersen M, Kyula J, et al. BRAF- and MEK-targeted small molecule inhibitors exert enhanced antimelanoma effects in combination with oncolytic reovirus through ER stress[J]. Mol Ther, 2015, 23(5):931-942.
[9] Kyula JN, Khan AA, Mansfield D, et al. Synergistic cytotoxicity of radiation and oncolytic Lister strain vaccinia in (V600D/E)BRAF mutant melanoma depends on JNK and TNF-alpha signaling[J]. Oncogene,2014, 33(13):1700-1712.
[10] Engeland CE, Grossardt C, Veinalde R, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy[J]. Mol Ther, 2014, 22(11):1949-1959.
[11] Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy[J]. Sci Transl Med, 2014,6(226):226ra32.
[12] 115. Kyle MP, William RM, Kyle PC, et al. The evolution and future of targeted cancer therapy: from nanoparticles, oncolytic viruses, and oncolytic bacteria to the treatment of solid tumors. Nanomaterials (Basel) 2021;11(11):3018. doi: 10.3390/nano11113018.
[13] 116. Tong M, Xiyu L, Yiqun L, et al. Aptamer-based biosensors and application in tumor theranostics. Cancer Sci. 2022;113(1):7–16. doi: 10.1111/cas.15194.
[14] 117. Briolay T, Petithomme T, Fouet M, et al. Delivery of cancer therapies by synthetic and bio-inspired nanovectors. Mol Cancer. 2021;20(1):1–24. doi: 10.1186/s12943-021-01346-2.
[15] 118. Robles-Planells C, Sánchez-Guerrero G, Barrera-Avalos C, et al. Chitosan-based nanoparticles for intracellular delivery of ISAV fusion protein cDNA into melanoma cells: a path to develop oncolytic anticancer therapies. Mediators Inflamm. 2020;2020.