- E-mail:BD@ebraincase.com
- Tel:+8618971215294
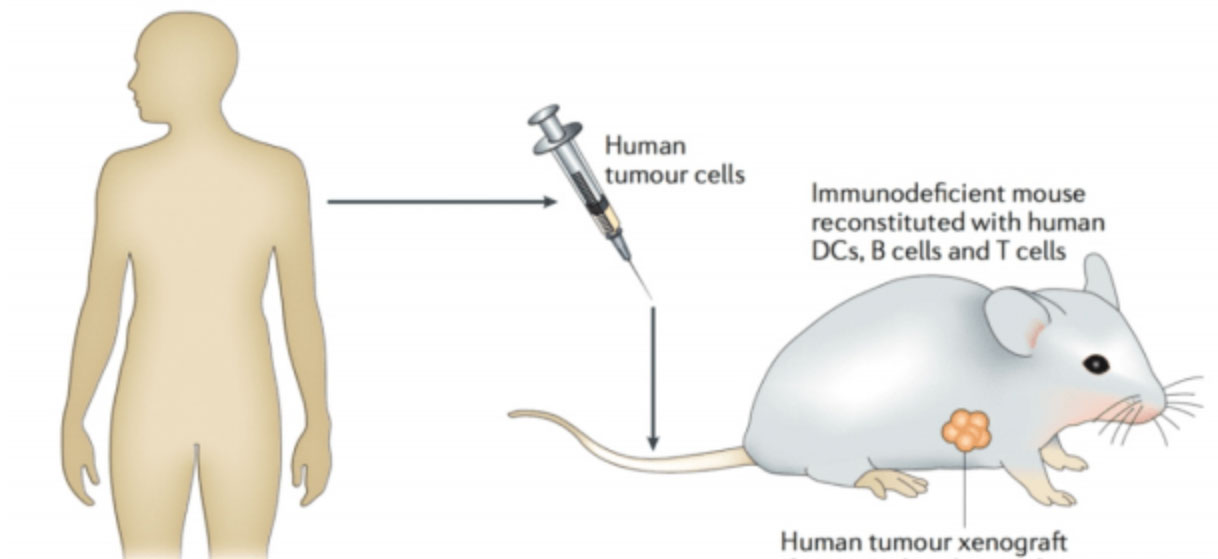
In order to evaluate the effects of cancer therapy in preclinical and clinical studies, the establishment of ideal tumor animal models provides a good alternative to clinical trials and is an important part of anti-tumor research. The establishment of tumor animal model can evaluate the efficacy of anti-tumor immunotherapy, serve as a screening model for anti-tumor drugs, provide a powerful tool for the study of tumor pathogenesis and metastasis, and enable researchers to understand the mechanism of clinical drug action, which plays an important role in the research and development of oncology and anti-tumor drugs. With the deepening of the understanding of tumor and the development of experimental zoology, as well as the application of some new techniques, the study of tumor animal models has also made important progress. The establishment of tumor animal model can be used to: (1) evaluate the efficacy of anti-tumor immunotherapy; (2) As a screening model for anti-tumor drugs; (3) To provide a better research platform for tumor metastasis research; (4) To provide a good experimental tool for the development of anti-metastatic drugs. Braincase Biotechnology Co., Ltd. provides: human gastric cancer, human liver cancer, human lung cancer, breast cancer, pancreatic cancer, prostate cancer and other human or mouse tumor cell tumorigenesis experiment, and related drug sensitivity analysis experiment; To provide customers with tumor in situ implantation, tumor metastasis model construction, induced tumor model construction, drug sensitivity analysis and other related services.
According to the transplantation method of tumor animal model, tumor model can be divided into subcutaneous transplantation tumor, subcapsular transplantation tumor, orthotopic transplantation tumor and blood transplantation tumor.
| neoplasms histologic type |
cell line | scope of application |
| breast cancer | MDA-MB-231 | Balb/c nude、C-NKG/in situ |
| colon cancer | Colo205, HT29 | Balb/c nude、C-NKG/subcutaneous |
| HCT116, SW480 | Balb/c nude、NOD-Scid、C-NKG/subcutaneous | |
| SW620 | NOD-Scid、C-NKG/subcutaneous | |
| lung cancer | NCI-H1299, NCI-H1975, NCI-H460 | Balb/c nude、C-NKG/subcutaneous |
| PC-9 | SCID-Beige、C-NKG/subcutaneous | |
| HCC827 | Balb/c nude、C-NKG/subcutaneous | |
| A549 | Balb/c nude、NOD-Scid、C-NKG/subcutaneous | |
| lymphoma | Raji | NOD-Scid、C-NKG/subcutaneous,caudal vein |
| melanoma | A375 | Balb/c nude、C-NKG/subcutaneous |
| ovarian cancer | OVCAR3, SK-OV-3 | Balb/c nude、NOD-Scid、C-NKG/subcutaneous |
| pancreatic cancer | PanC-1 | Balb/c nude、C-NKG/subcutaneous |
| AsPC-1, BxPC-3 | Balb/c nude、NOD-Scid、C-NKG/subcutaneous | |
| gastric cancer | HGC-27 | Balb/c nude、NOD-Scid、C-NKG/subcutaneous |
| liver cancer | Hep G2 | Balb/c nude、NOD-Scid、C-NKG/subcutaneous |
| HuH7 | NOD-Scid、C-NKG/subcutaneous | |
| NCI-H1299 | Balb/c nude、C-NKG/subcutaneous | |
| cervical cancer | Hela | Balb/c nude、NOD-Scid、C-NKG/subcutaneous |
| prostatic cancer | 22RV1 | NOD-Scid、C-NKG/subcutaneous |
literature citation