- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Herpes simplex virus (HSV) is one of the most widely used oncolytic viruses (OV) in clinic. HSV is a double-stranded DNA virus that can encode 84 different polypeptides, and its genome length is 152Kb. The HSV genome consists of two unique sequences: a long segment (Unique Long (UL)) and a short segment (Unique Short (US)), both ends of which are terminal inverted repeats connected by internal inverted repeats.
The HSV viral genes are divided into three categories according to the timing of their expression: early or alpha genes, middle or beta genes, and late or gamma genes. These oncolytic genes encode proteins that promote viral replication and elicit host immune responses [1]. Among them, the main function of the α gene is to regulate the transcription of the β and γ genes. After DNA replication, the gamma gene mainly encodes the structural components of the virion. Viral DNA replication and protein synthesis lead to lysis of cancer cells, and subsequently, progeny viruses are released to infect adjacent cancer cells, expanding the ability to kill cancer cells.
HSV can be exerted locally (peritoneal or pleural infusion and intratumoral injection) and systemically (vascular delivery). Intratumoral injection can limit its delivery to the tumor site only. Some physical barriers such as extracellular matrix and host immune response against HSV can inhibit viral replication.
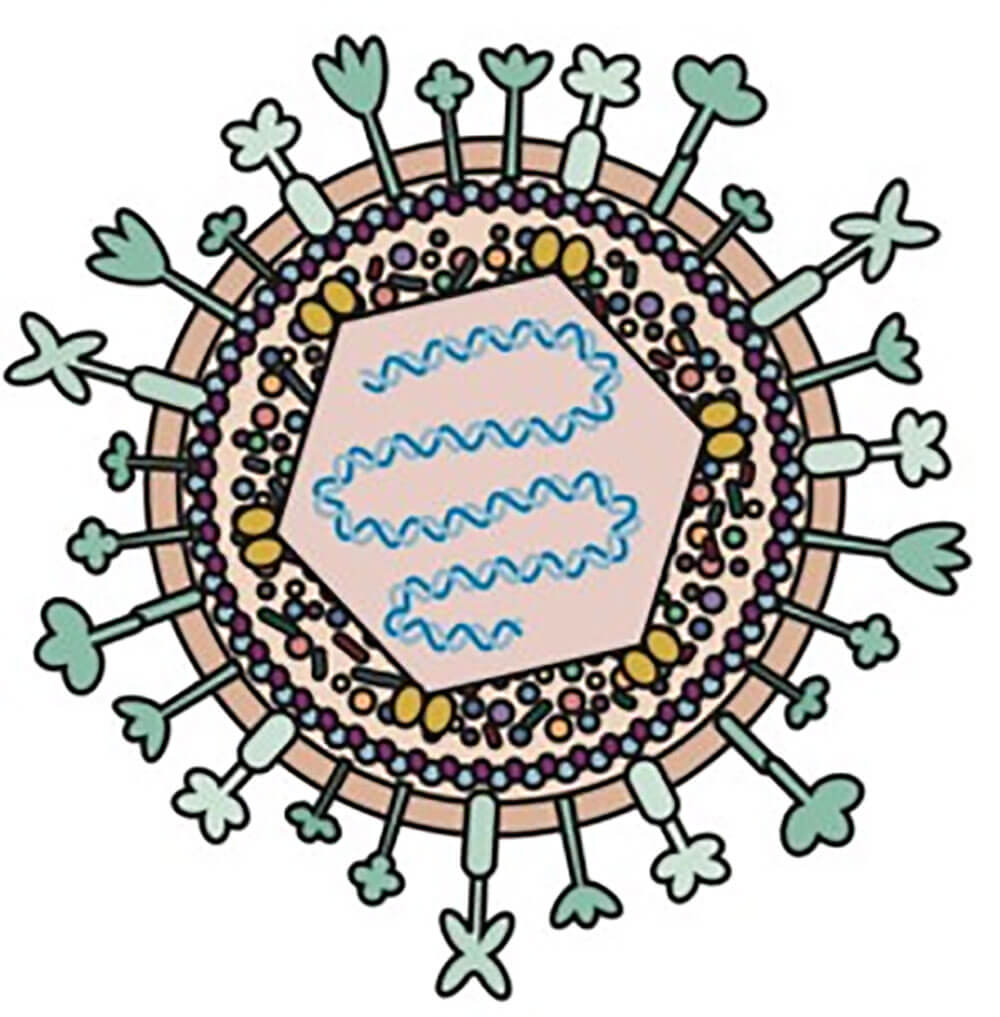
Figure 1. Schematic diagram of herpes simplex virus type 1 (HSV-1)
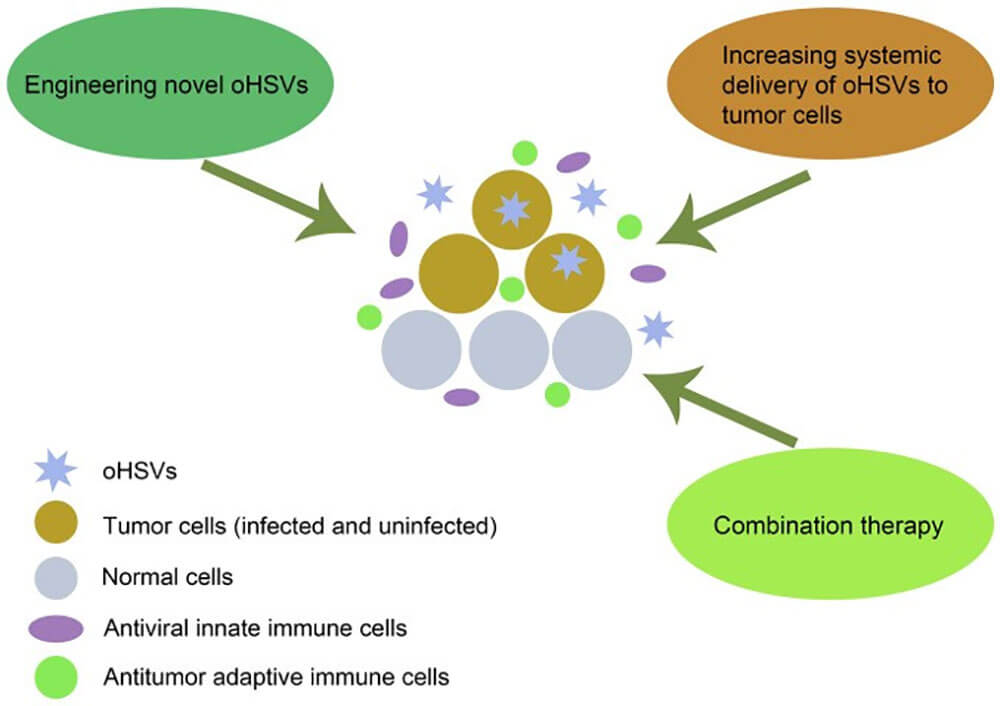
Figure 2: Schematic diagram of the destruction mechanism of oHSVs to destroy tumor cells and various strategies to improve its efficacy doi:10.3892/ol.2021.12771
oHSV directly destroys tumor cells and stimulates anti-tumor immune responses by selectively replicating in tumor cells and evading antiviral immunity, which is the main mechanism for oHSV to kill cancer cells. Multiple strategies can improve the efficacy of oHSV in cancer therapy, including designing novel oHSV, increasing systemic delivery of oHSV to tumor cells, and combination therapy.
Approved by the US Food and Drug Administration (FDA) in 2015, talimogene laherparepvec (T-VEC) for the treatment of melanoma is an effective clinical drug for HSV-1 gene modification, and it is also an important clinical application of OV. Breakthrough [5-6]. The official name of T-VEC is OncovexGM-CSF, which is a genetically engineered strain of HSV-1, which has lost two copies of the ICP34. colonystimulating factor, GM-CSF) encoding gene. OncovexGM-CSF exerts antitumor effects through direct tumor lysis and induction of tumor-specific secondary immune responses, including enhancement of antigen-specific T cell responses, suppression of levels of CD4+Tregs, CD8+Ts, and myeloid-derived suppressor immune cells (MDSCs) [7]. For patients with unresectable advanced melanoma (OPTIM clinical trial) phase III clinical trial, in the phase III clinical trial of advanced melanoma, the objective response rate of OncovexGM-CSF was 26%, the sustained response rate was 16%, complete The response rate was 11% [8].
In 2021, the third-generation oncolytic herpes simplex virus vector G47Δ was approved by the Japanese Ministry of Health, Labor and Welfare (WHLW) for the treatment of glioma. In order to improve its anti-tumor activity, the ICP34.5 gene was knocked out to eliminate the neurotoxicity of the virus, and the 312bp α47 gene was knocked out to improve the replication and reproductive capacity of the virus [9-10]. At the same time, inserting the ECOL-I LacZ gene into the ICP6 (UL39) region inactivates nucleotide reductase (RR), allowing the virus to reproduce only in tumor cells, thereby improving its oncolytic effect [9]. G47Δ shows strong replication ability in various cancers, effectively induces specific anti-tumor immunity, and shows high safety [11]. G47Δ can kill cancer cells very effectively. In a phase II clinical trial involving patients with recurrent glioma, each patient received tumor stereotaxic injection of G47Δ, once every 4 weeks, up to 6 times, an interim analysis of clinical trial data showed that 1-year The survival rate was 92.3%. There were only 2 patients with relevant serious adverse events and both were grade 2 fever. G47Δ was designated as a SAKIGAKE (Breakthrough Therapy) product by the Japanese government and further designated as an orphan drug for malignant glioma, which is the second approved oncolytic herpes simplex virus drug after T-VEC and the first in the world OV drugs approved for the treatment of malignant glioma [10].
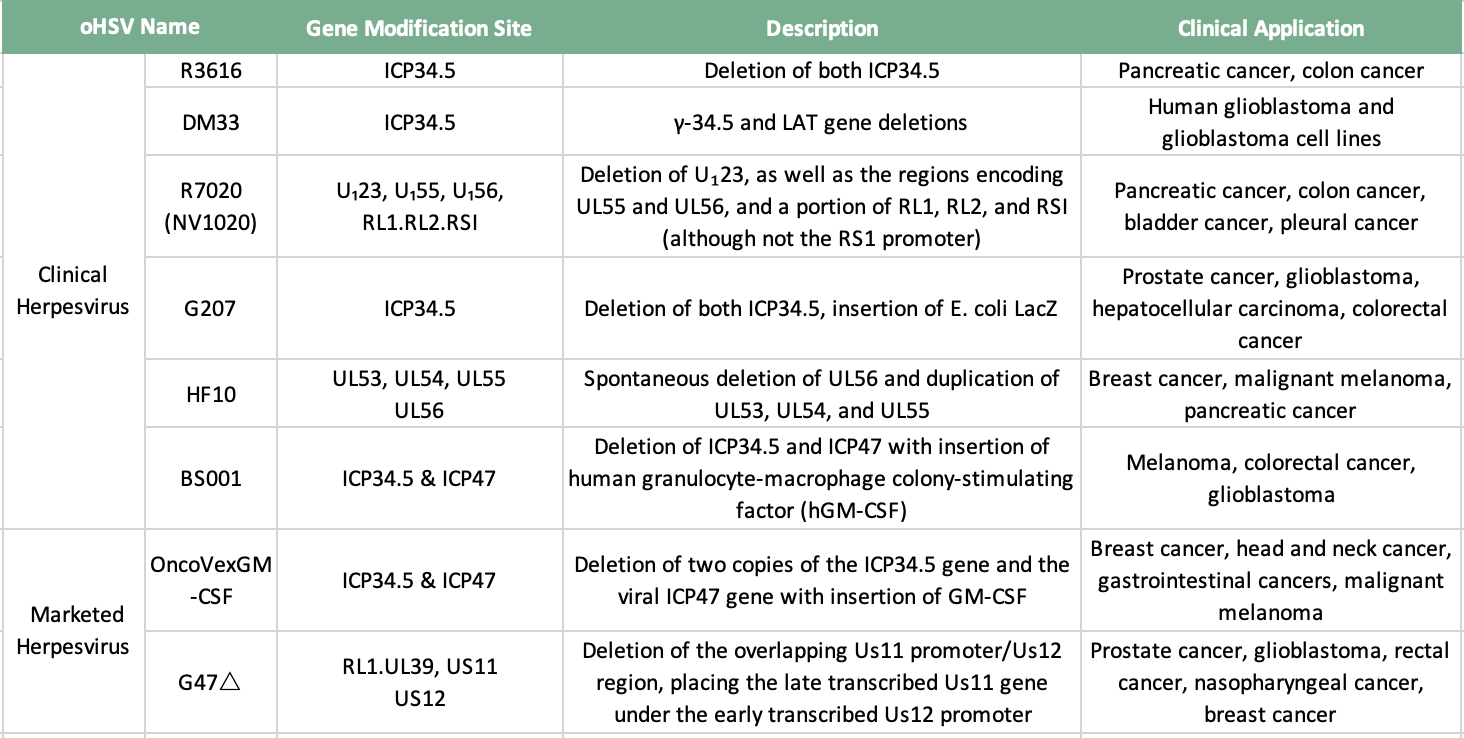
Table 1: Marketable and Clinical Herpesviruses
Currently, oHSV in clinical research is mainly type 1, and type 2 oHSV also shows strong research potential, and companies have begun to deploy.
R3616 is an HSV-1(F)-derived recombinant virus that lacks two copies of the ICP34.5 gene [12]. Host protein kinase R (protein kinase R, PKR) senses various stressors such as viral dsRNA and prevents the synthesis of viral proteins. One of the functions of ICP34.5 is to mediate the dephosphorylation of eIF2a and release the inhibition of protein synthesis. Thus, R3616 targets cancer cells characterized by uncontrolled protein synthesis. Compared with conventional therapy, R3616 can induce a more effective host anti-tumor immune response. In a mouse colon cancer model, R3616 can induce a large number of infiltrating T cells, macrophages and dendritic cells [13]. R3616 combined with gemcitabine in the treatment of advanced pancreatic cancer can produce a synergistic effect [14].
DM33 is a recombinant HSV-1OV with double deletion of ICP34.5 and LAT genes. Different from DLSPTK, the mutation of DM33 based on McKrae strain has stronger proliferative ability and induces stronger antitumor effect. In a nude mouse model bearing U-87MG human glioma intracranially, the survival time of mice treated with DM33 was prolonged, and histological analysis showed that the DM33 treatment group accelerated tumor cell lysis. The safety of DM33 lies in its attenuating effect on neuronal cells, astrocytes and endothelial cells.
NV1020 is an attenuated recombinant virus derived from HSV-1 by replacing five HSV-1 genes with regions of the HSV-2 genome encoding several viral glycoproteins [15]. In addition to RL1 loss, UL56 loss most likely further reduces neurovirulence because pUL56 is associated with neuron-specific kinesin (Kinesin Family, Member1A, KIF1A), a protein involved in the axonal transport of synaptic vesicle precursors , and further attenuated its toxicity by deleting the promoters of the TK gene and the gene UL24[16]. These genetic modifications allowed NV1020 to be greatly attenuated and allowed to spread only in tumor cells. The safety and efficacy of NV1020 have been tested in pancreatic cancer, colon cancer, bladder cancer and pleural cancer. Combined with NV1020's cytotoxicity and tumor targeting, it has been applied in Phase II and Phase III clinical trials.
G207 is the first oHSV-1 to be used in clinical trials. It deletes two copies of ICP34.5 and ICP6 and inserts E. coli LacZ. The TK derived from HSV is complete in G207, and its anti-tumor effect can be enhanced by combination therapy [17]. G207 induces systemic anti-tumor immunity, and the effect of anti-tumor immunity is related to the enhancement of the activity of cytotoxic T lymphocytes [18]. G47Δ is a kind of HSV-1 mutant derived from G207, which deletes the non-essential gene α47 and promotes the replication of G47Δ.
HF10 has a 3.9 kbp deletion at the right end of UL and UL/irL binding, resulting in loss of UL56 expression. Compared with wild-type HSV-1, HF10 is less virulent and has more advanced replication ability. Its safety and efficacy have been confirmed in many animal models, and it has been used to treat ovarian cancer, malignant melanoma, breast cancer and Clinical trials in pancreatic cancer.
BS001 is the first oHSV-2 for clinical use. Promote the selective replication of the virus in tumor cells by deleting ICP34.5, and enhance safety; delete the virulence gene ICP47, reduce immunosuppression, promote virus replication, and enhance anti-tumor efficacy: insert human granulocyte-macrophage colony-stimulating factor ( hGM-CSF) expression sequence can induce the differentiation, proliferation and maturation of tumor and surrounding dendritic cell (DC) precursors, and at the same time enhance the antigen presentation of DC to activate immune killer cells in vivo, which helps to induce local and systemic anti-tumor immune response. An oncolytic virus drug that has been certified as an orphan drug by the US FDA.
At present, most of the genetic modification of HSV is based on the inactivation of the ICP6 gene (UL39) and the deletion of the ICP34.5 gene [19]. The ICP6 protein is the large subunit of ribonucleotide reductase essential for viral DNA replication. ICP6-mutated HSV can only replicate in rapidly dividing cells such as tumor cells, which can improve its tumor targeting. The viral gene ICP34.5 is the major neurovirulence gene in HSV [14]. Phosphorylation of the α-subunit of eukaryotic initiation factor-2α (eIF-2α) blocks host protein synthesis, whereas expression of the ICP34.5-encoded protein ICP34.5 results in dephosphorylation of eIF-2α , to relieve its inhibitory effect on protein synthesis. The RAS/mitogen-activated protein kinase pathway is normally activated in cancer cells, which allows ICP34.5-deficient HSV to selectively replicate in cancer cells. As a result of genetic modification, ICP6/ICP34.5-deleted HSV has low pathogenicity to normal tissues and high oncolytic ability to tumor cells [20].
At present, the gene design of HSV-1 generally uses virus strains or plasmids containing the virus genome, and undergoes multiple rounds of recombination through homologous recombination technology or bacterial artificial chromosome (BAC) technology to obtain the target virus.
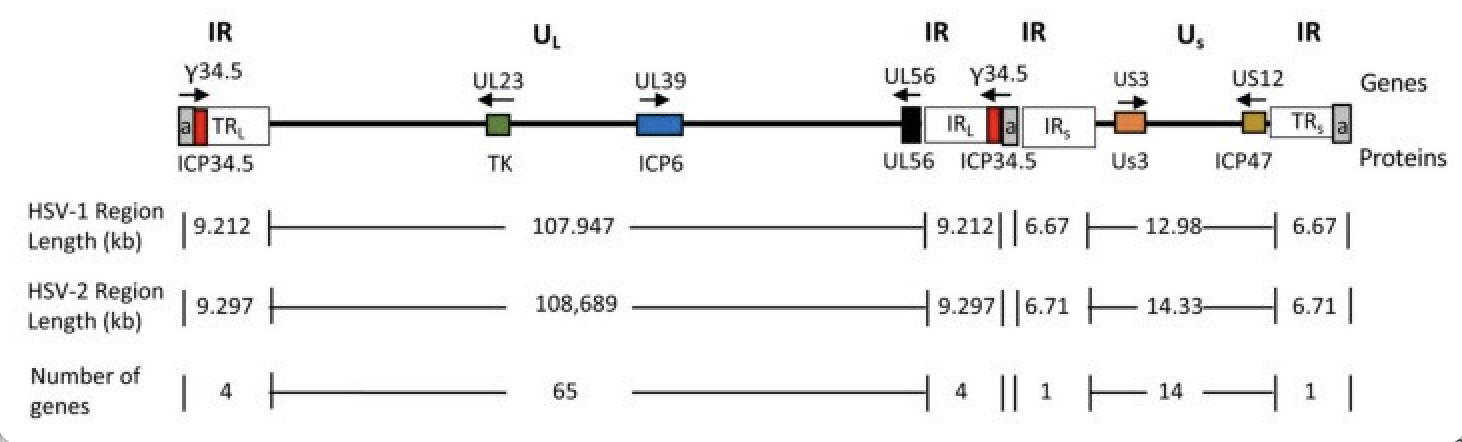
Figure 3. Schematic diagram of the HSV genome doi:10.3390/v13091740
The number of sequence repetitions (a, gray) is variable. Tumor-specifically associated genes are shown as colored boxes with their names shown above the genomic line; gene product names are shown below the genomic line. Arrows indicate direction of transcription.
Virus is an efficient gene delivery system, and OV can produce more effective anti-tumor effect through genetic modification. As a carrier, HSV-1 can introduce large therapeutic genes into its viral genome, and has been successfully applied to deliver various immune regulatory molecules, including interleukin-12 (Interleukin-12, IL-12), IL- 24 (Interleukin-24, IL-24), IL-4 (Interleukin-4, IL-4), IL-18 (Interleukin-18, IL-18) and interferon α (Interferon α, IFN-α). These transferred therapeutic genes inhibit tumor growth by stimulating local inflammation or eliciting host immune responses. IL-24, IL-4, and IFN-γ have been shown to selectively induce tumor cell apoptosis by inhibiting tumor angiogenesis, regulating T helper cell maturation, and modulating the tumor microenvironment [27]. Solid tumors limit the effectiveness of immunotherapy in different ways, such as the secretion of immunosuppressive cytokines and the expression of immunosuppressive ligands that suppress the function of antitumor T cells. OV, combined with PD-L1 or PD-L2 inhibitors, blocks the programmed cell death protein-1 (PD-1) signaling pathway, relieves the inhibition of T cells, and has been obtained in many types of cancer trials Success [28].
The current standard of clinical treatment for patients with glioblastoma (glioblastoma multiforme, GBM) is temozolomide (TMZ) chemotherapy combined with maximum safe resection and radiotherapy. Recurrent disease is generally less effective with this treatment approach. Studies have shown that oHSV-TRAIL (oHSV-TNF related apoptosis inducing ligand) is expected to overcome the ability of GBM treatment resistance and relapse, providing a basis for its use in clinical trials for GBM treatment [29].
At this stage, various types of OV have been clinically tested. Understanding the interactions between OVs, the tumor microenvironment and the immune system is important for engineering OVs and improving their safety and antitumor properties. There is no definite answer to the mechanism of enhancing OV oncolysis in vivo and the factors that affect the spread of OV in the tumor microenvironment. In addition, the clinical anti-tumor effect of the combination of OV and conventional therapy needs to be confirmed by more experiments. Modification of HSV with therapeutic transgenes is a promising strategy in cancer therapy that can be used to complement the inadequacies of conventional therapies. With the continuous advancement of various studies, there is reason to believe that OV will play an important role in tumor treatment.
literature citation
[1]Conrady CD, Drevets DA, Carr DJJ. Herpes Simplex Type I (HSV-1) Infection of the Nervous System: Is an Immune Response a Good Thing? Journal of neuroimmunology. 2010;220(1-2):1-9. doi:10.1016/j.jneuroim.2009.09.013
[2]Zeyaullah M, Patro M, Ahmad I, et al. Oncolytic viruses in the treatment of cancer: a review of current strategies. Pathology oncology research: POR. 2012;18(4):771-781. doi:10.1007/s12253-012-9548-2
[3]Dai MH ., Zamarin D, Gao SP, et al. Synergistic action of oncolytic herpes simplex virus and radiotherapy in pancreatic cancer cell lines. The British Journal of Surgery. 2010;97(9):1385-1394. doi:10.1002/bjs.7124
[4]Watanabe D. Medical application of herpes simplex virus. Journal of Dermatological Science. 2010;57(2):75-82. doi:10.1016/j.jdermsci.2009.10.014
[5]Killock D. T-VEC oncolytic viral therapy shows promise in melanoma. Nature Reviews Clinical Oncology. 2015;12(8):438-438. doi:10.1038/nrclinonc.2015.106
[6]Johnson DB, Puzanov I, Kelley MC. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy. 2015;7(6):611-619. doi:10.2217/imt.15.35
[7]Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and Distant Immunity Induced by Intralesional Vaccination with an Oncolytic Herpes Virus Encoding GM-CSF in Patients with Stage IIIc and IV Melanoma. Annals of Surgical Oncology. 2009;17(3):718-730. doi:10.1245/s10434-009-0809-6
[8]Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Journal of Clinical Oncology. 2015;33(25):2780-2788. doi:10.1200/jco.2014.58.3377
[9]Wang JN, Liu RB, Jun-Jie LI, Abdalwalithabit M. Oncolytic herpes simplex virus vectors for the treatment of human breast cancer. Chinese Journal of Breast Disease(Electronic Edition). Published online 2009.
[10]INO Y, TODO T. CLINICAL DEVELOPMENT OF A THIRD-GENERATION ONCOLYTIC HSV-1 (G47Δ) FOR MALIGNANT GLIOMA. Gene Therapy and Regulation. 2010;05(01):101-111. doi:10.1142/s1568558610000185
[11]Todo T. ATIM-14. RESULTS OF PHASE II CLINICAL TRIAL OF ONCOLYTIC HERPES VIRUS G47Δ IN PATIENTS WITH GLIOBLASTOM. Neuro-oncology. 2019;21(Suppl 6):vi4-vi4.
[12]12. Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of Herpes Simplex Virus-1 Neurovirulence to γ134.5, a Gene Nonessential for Growth in Culture. Science. 1990;250(4985):1262-1266. doi:10.1126/science.2173860
[13]Shirota T, Kasuya H, Kodera Y, et al. Oncolytic Herpes Virus Induces Effective Anti-Cancer Immunity against Murine Colon Cancer. Hepatogastroenterology. 2011;58(110-111). doi:10.5754/hge11168
[14]Watanabe I, Kasuya H, Nomura N, et al. Effects of tumor selective replication-competent herpes viruses in combination with gemcitabine on pancreatic cancer. Cancer Chemotherapy and Pharmacology. 2007;61(5):875-882. doi:10.1007/s00280-007-0567-8
[15]Loret S, Guay G, LippéR. Comprehensive Characterization of Extracellular Herpes Simplex Virus Type 1 Virions. Journal of Virology. 2008;82(17):8605-8618. doi:10.1128/jvi.00904-08
[16]Koshizuka T, Kawaguchi Y, Nishiyama Y. Herpes simplex virus type 2 membrane protein UL56 associates with the kinesin motor protein KIF1A. Journal of General Virology. 2005;86(3):527-533. doi:10.1099/vir.0.80633-0
[17]Kroeger KM, Muhammad AKMG, Baker GJ, et al. Gene therapy and virotherapy: novel therapeutic approaches for brain tumors. Discovery Medicine. 2010;10(53):293-304. Accessed November 18, 2022. https://pubmed.ncbi.nlm.nih.gov/21034670/
[18]Todo T. “Armed” oncolytic herpes simplex viruses for brain tumor therapy. Cell Adhesion & Migration. 2008;2(3):208-213. doi:10.4161/cam.2.3.6353
[19]Eager RM, Nemunaitis J. Clinical development directions in oncolytic viral therapy. Cancer Gene Therapy. 2011;18(5):305-317. doi:10.1038/cgt.2011.7
[20]Walker JD, Sehgal I, Kousoulas KG. Oncolytic herpes simplex virus 1 encoding 15-prostaglandin dehydrogenase mitigates immune suppression and reduces ectopic primary and metastatic breast cancer in mice. Journal of Virology. 2011;85(14):7363-7371. doi:10.1128/JVI.00098-11
[21]Buijs PR, Verhagen JH, van Eijck CH, van den Hoogen BG. Oncolytic viruses: From bench to bedside with a focus on safety. Human Vaccines & Immunotherapeutics. 2015;11(7):1573-1584. doi:10.1080/21645515.2015.1037058
[22]Zhou G, Roizman B. Construction and properties of a herpes simplex virus 1 designed to enter cells solely via the IL-13alpha2 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(14):5508-5513. doi:10.1073/pnas.0601258103
[23]Menotti L, Cerretani A, Hengel H, Campadelli-Fiume G. Construction of a Fully Retargeted Herpes Simplex Virus 1 Recombinant Capable of Entering Cells Solely via Human Epidermal Growth Factor Receptor 2. Journal of Virology. 2008;82(20):10153-10161. doi:10.1128/jvi.01133-08
[24]Zhou G, Ye GJ ., Debinski W, Roizman B. Engineered herpes simplex virus 1 is dependent on IL13R 2 receptor for cell entry and independent of glycoprotein D receptor interaction. Proceedings of the National Academy of Sciences. 2002;99(23):15124-15129. doi:10.1073/pnas.232588699
[25]Menotti L, Cerretani A, Campadelli-Fiume G. A Herpes Simplex Virus Recombinant That Exhibits a Single-Chain Antibody to HER2/neu Enters Cells through the Mammary Tumor Receptor, Independently of the gD Receptors. Journal of Virology. 2006;80(11):5531-5539. doi:10.1128/jvi.02725-05
[26]Grandi P, Fernandez J, Szentirmai O, et al. Targeting HSV-1 virions for specific binding to epidermal growth factor receptor-vIII-bearing tumor cells. Cancer Gene Therapy. 2010;17(9):655-663. doi:10.1038/cgt.2010.22
[27]Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Molecular Therapy: The Journal of the American Society of Gene Therapy. 2010;18(2):251-263. doi:10.1038/mt.2009.283
[28]Chen CY, Wang PY, Hutzen B, et al. Cooperation of Oncolytic Herpes Virotherapy and PD-1 Blockade in Murine Rhabdomyosarcoma Models. Scientific Reports. 2017;7(1). doi:10.1038/s41598-017-02503-8
[29]Jahan N, Lee JM, Shah K, Wakimoto H. Therapeutic targeting of chemoresistant and recurrent glioblastoma stem cells with a proapoptotic variant of oncolytic herpes simplex virus. International Journal of Cancer. 2017;141(8):1671-1681. doi:10.1002/ijc.30811