- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Rabies Virus(It is also commonly abbreviated as RbV) is an enveloped negative-sense single-stranded RNA virus. It is ~75 nm in diameter and 180 nm in length. It is bullet-shaped with a genome of ~12 kb and is highly neurotropic. Its replication is accomplished by a self-encoded RNA polymerase complex(Figure 1).
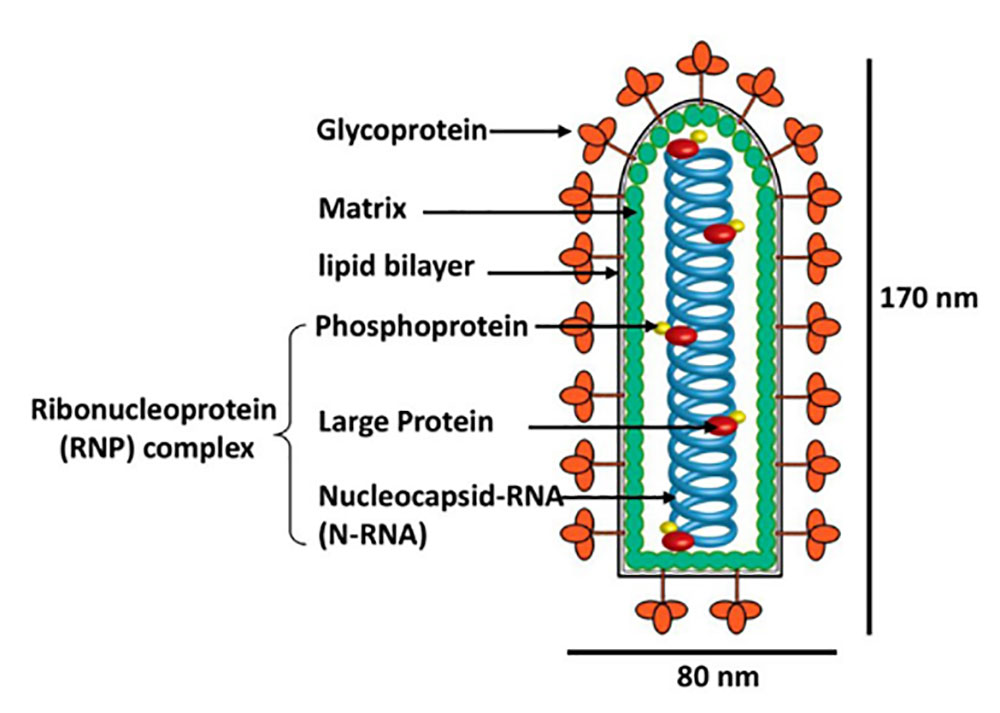
Fifure 1. Schematic diagram of RV particles (picture from whelanlab)
In the host body, RV glycoprotein G mediates the binding of virus particles to cell surface receptors and enters cells through endocytosis. Subsequent pH-mediated membrane fusion promotes the release of the RV genome into the cytoplasm. The polymerase complex composed of L, N, and P packaged in the infectious virus particle uses the genomic negative-strand RNA as a template to initiate transcription expression, replication, assembly, and budding to produce progeny viruses. (As shown in Figure 2)
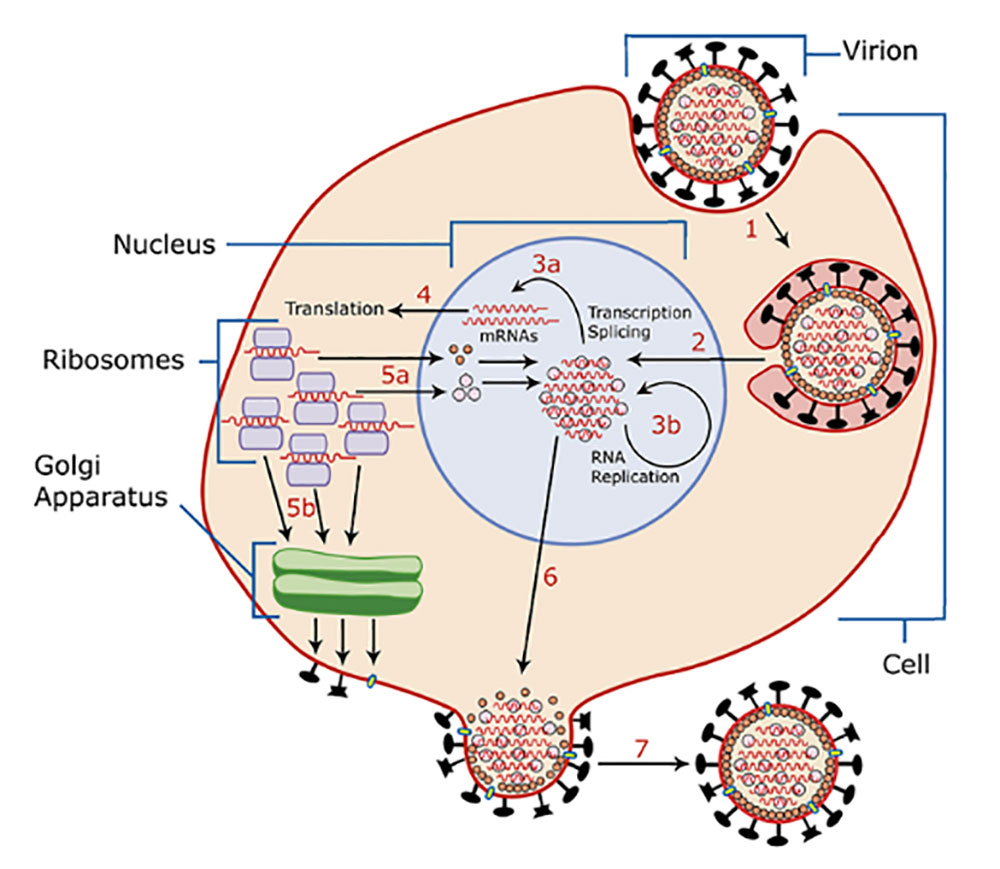
Figure 2. Schematic diagram of rabies virus life cycle (picture from Wikipedia)
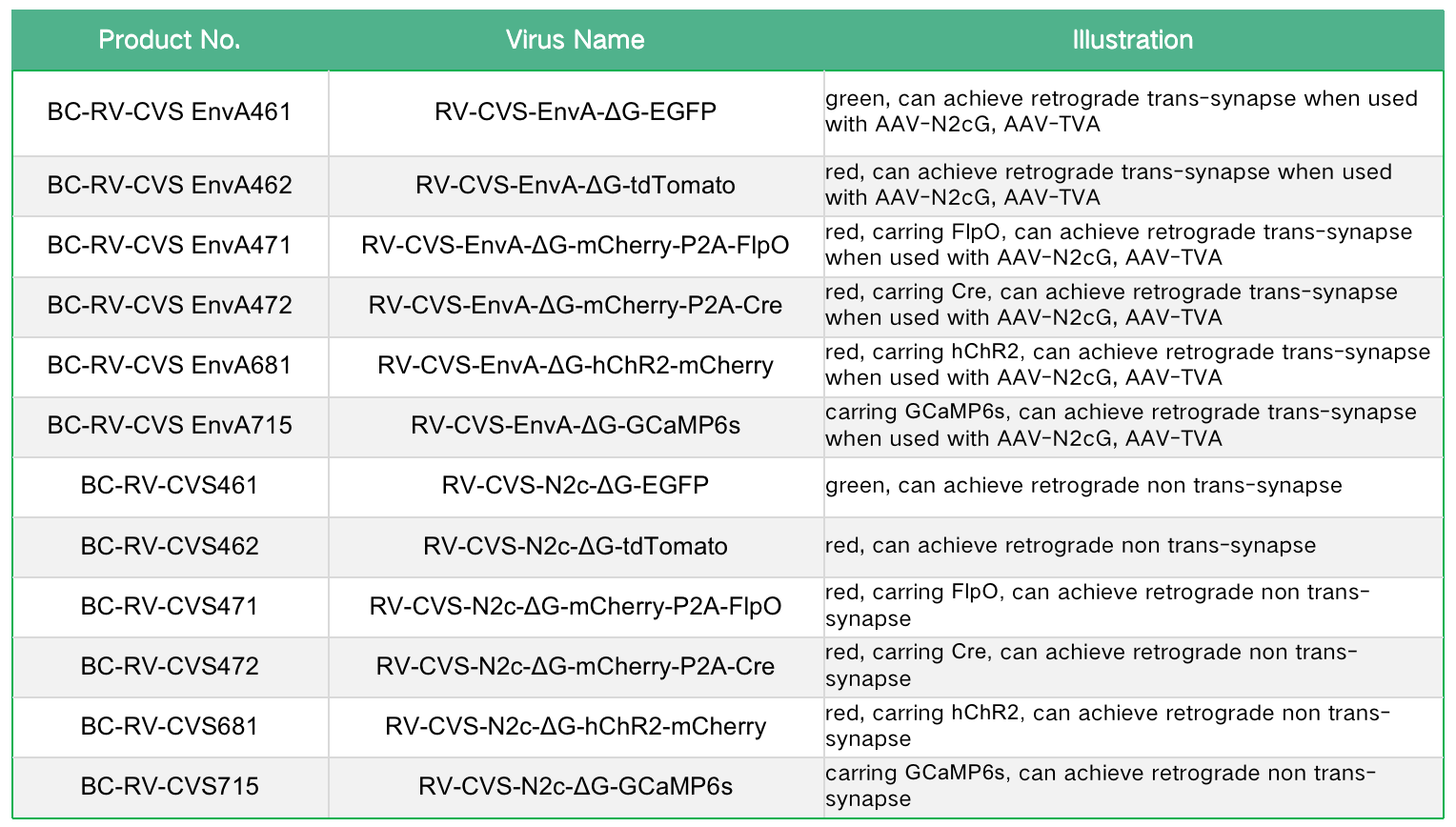
Wild-type RV is the causative agent of zoonotic rabies, has propagation properties in the nervous system, and is highly pathogenic to both humans and animals. Therefore, It has been modified to be safer for applications in the laboratory by deletion of the envelope glycoprotein gene (RV-ΔG) and then used as a tracer for retrograde infection of projection neurons through their axon terminals, targeted infection of genetically specified neurons, or trans-monosynaptic tracing of direct input neurons after further modifications. our company's RV products are modified from the rabies virus vaccine strain (SAD B19 or CVS-N2c) and the replication-deficient RV lacking G protein (RV-SAD-ΔG/RV-CVS-ΔG) as the backbone. With the maturity of reverse genetics methods, modified RVs can be used for neural circuit research. After RV infects the central system, it mainly labels neurons and almost no glial cells; the infected neurons rarely undergo obvious lesions or lysis within a certain period of time (7-12 days). Currently, RV systems can be divided into retrograde tracing systems and retrograde trans-monosynaptic tracing systems.
The envelope glycoprotein (G) of RV is an essential protein for retrograde transsynapsis. RV lacking G protein (RV-SAD-ΔG) will lose its transsynaptic ability, but its replication and transcription are not affected ( Sustainably express foreign genes in high abundance), therefore, the RV (RV-SAD-B19G-ΔG) encapsulating the SAD B19G protein carries the reporter gene and can retrogradely label the fine morphology of neurons with high brightness.
The second generation retrograde tracing system uses the CVS-N2c strain, which has lower neurotoxicity and stronger trans-synaptic ability. The second generation retrograde tracing RV-CVS-N2C(G)-ΔG product with the same titer showed higher retrograde gene transduction efficiency than the first generation RV-SAD-B19G-ΔG, with lower neurotoxicity.
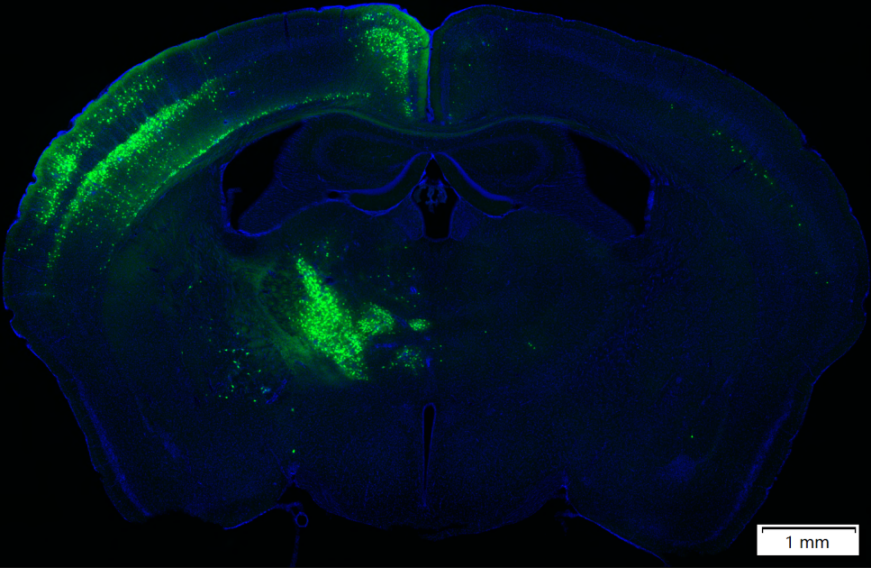
Figure 3. RV-CVS-N2c-ΔG-EGFP (Tested by Brain Case)
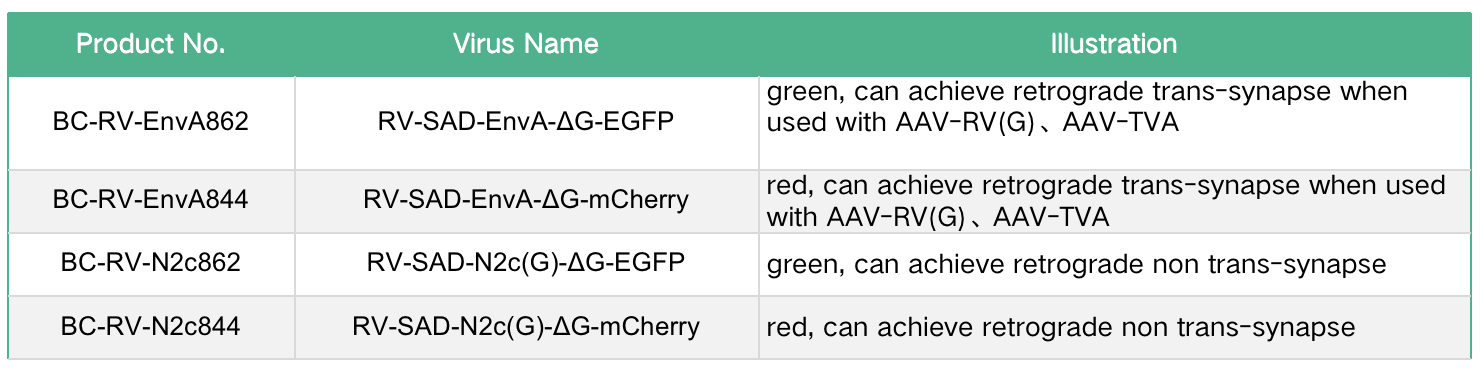
The recombinant aviansarcoma-leukosis virus (ASLV) outer membrane protein (EnvA) fusion protein is used to package the viral particles (RV-SAD-EnvA-ΔG) formed by the RV-SAD-ΔG genome, which can specifically recognize the EnvA receptor. Because mammalian neurons do not express TVA, only cells engineered to express TVA can be infected by RV-SAD-EnvA-ΔG. Using Cre transgenic mice combined with Cre-LoxP system to control AAV helper viruses (DIO-TVA and DIO-RVG) that express TVA and G proteins, it is possible to express TVA and G proteins only in specific types of neurons in specific regions, thereby utilizing RV-SAD -EnvA-ΔG realizes retrograde tracing direct inputs to specific cell types (Figure 3). On this basis, by carrying genes such as ChR2 and GCaMP in RV-SAD-EnvA-ΔG, functional manipulation and activity monitoring of the direct output network of specific types of neurons can be achieved.
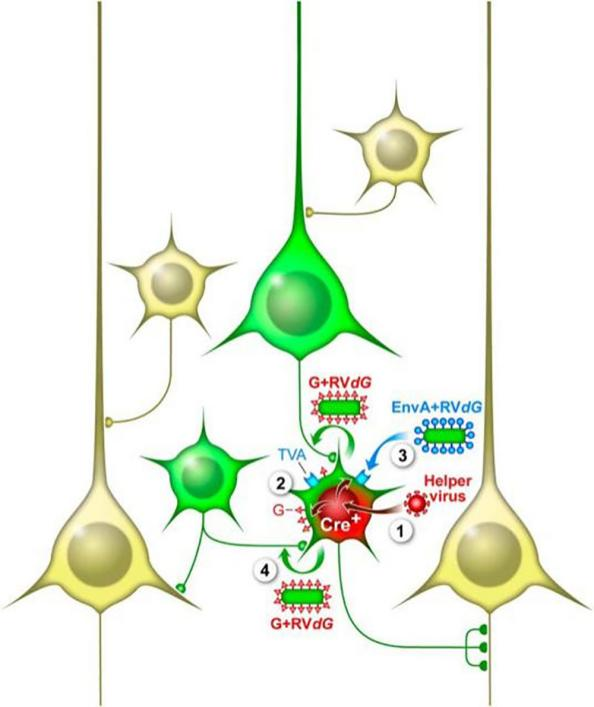
Figure 4. RV-SAD-EnvA-ΔG achieves retrograde transsynaptic tracing of neurons (Callaway, et al., The Journal of neuroscience: the official journal of the Society for Neuroscience, 2015)
The second-generation retrograde trans-monosynaptic tracing system uses the CVS-N2c strain and uses Cre transgenic mice combined with Cre-LoxP to control the AAV helper virus (DIO-TVA and DIO-N2cG) expressing TVA and G proteins, which can achieve only specific regions. TVA and G proteins are expressed in specific types of neurons, thus using RV-EnvA-ΔG to achieve retrograde transsynaptic tracing of specific types of neurons (the principle is the same as the first-generation reverse trans-single synapse system). Compared with the first-generation retrograde transsynaptic system, RV-CVS-EnvA-ΔG has lower neurotoxicity and stronger transsynaptic transmission ability.
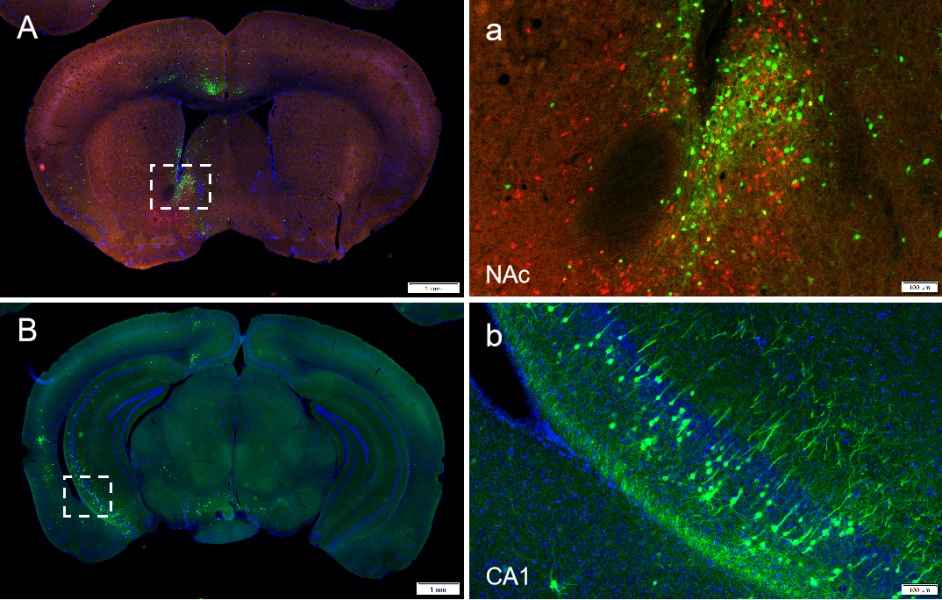
Figure 5. RV-CVS-EnvA-ΔG-EGFP (Tested by Brain Case)
1)The retrograde tracing system has been used as a tracer for retrograde infection of projection neurons through their axon terminals
2)The retrograde trans-monosynaptic system has been widely used to map the input network of specifc types of neurons
3)N2cG coated CVS-N2c-ΔG carrying ChR2 or GCaMP can be used to monitor the neural activity of projection neurons
Brain Case RV series products are divided into RV-CVS strain and SAD-B19 vaccine strain.
Experimental animals: PV-Cre, SST-Cre, VIP Cre adult male mice
Viruses used: RV-EnvA-GFP, AAV-DIO-TVA-hBFP and AAV-DIO-RG
Experimental methods and results: Briefly, the Cre-dependent AAV helper virus was injected into the mPFC of SST-Cre, PV-Cre or VIP-Cre mice. After 3weeks, the EnvA-coated RV expressing GFP and the RG-coated RV expressing DsRed were injected into the mPFC and ACB, respectively. The virus reversely traces the neurons in the hippocampus that project to the ACB area, and finally find the function in the hippocampus that regulates both mPFC and ACB areas. GABAergic neurons. (As shown in Figure 6)
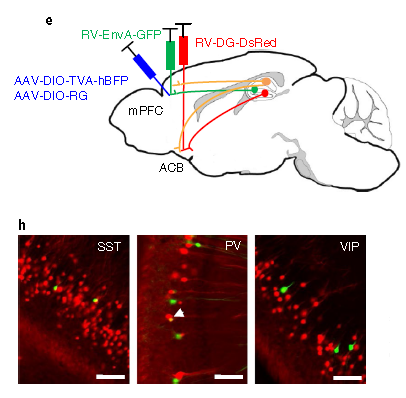
Figure 6. The strategy for labeling hippocampal pyramidal neurons that simultaneously target GABAergic neurons in the mPFC and ACB.
Experimental animals: C57BL/6 mice
Viruses used: RV-DG-DsRed and RV-DG-GFP, titer 10^8 TU
Experimental methods and results: 100 nl RV-DG-DsRed and RV-DG-GFP were injected into the NAC and mOT respectively. After 1 week, the samples were perfused and the neurons could be traced in the VTA. (As shown in Figure 7)
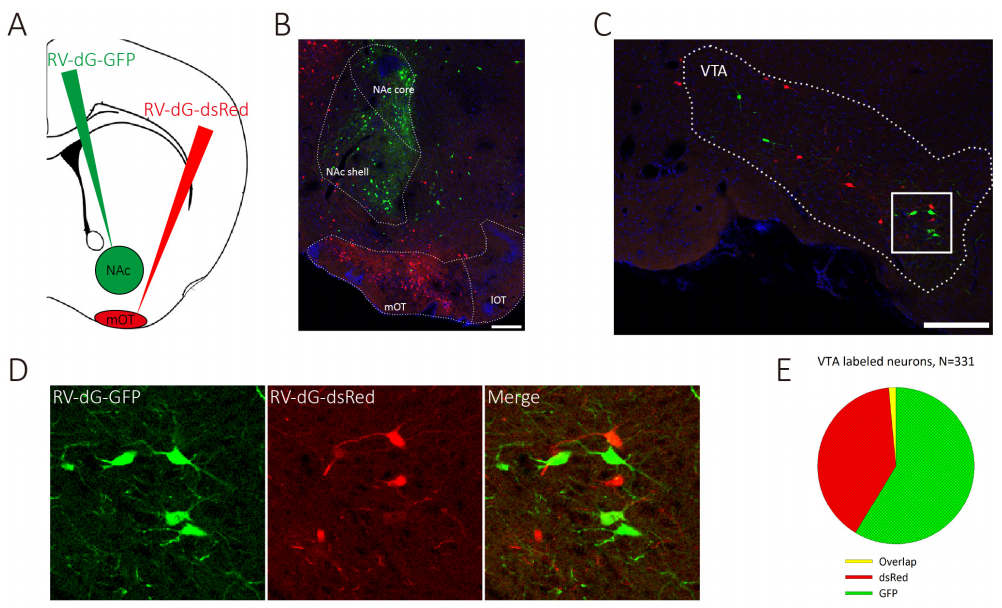
Figure 7. VTA DAergic neurons segregatedly innervate the NAc and the mOT.