- E-mail:BD@ebraincase.com
- Tel:+8618971215294
The realization of complex functions of the brain is inseparable from the efficient and specific information exchange and integration between neurons. Neurotransmitters, as important biological small molecules, participate in the information transmission process mediated by chemical synapses between neurons. Most of the known neurotransmitters have corresponding G protein-coupled receptors (GPCRs) as receptors. Therefore, GPCRs, as a type of natural endogenous receptors that can bind to neurotransmitters, are important for constructing genetically encoded neurotransmitters. The preferred backbone for mass sensors. The neurotransmitter sensor constructed based on the GPCR activation principle is named GRAB sensor. Compared with traditional direct measurement methods, GRAB neurotransmitter fluorescent sensors have better time resolution, higher molecular specificity, high sensitivity, high signal-to-noise ratio and spatial resolution, making them ideal for studying endogenous neurotransmitters. The release provides powerful tools.
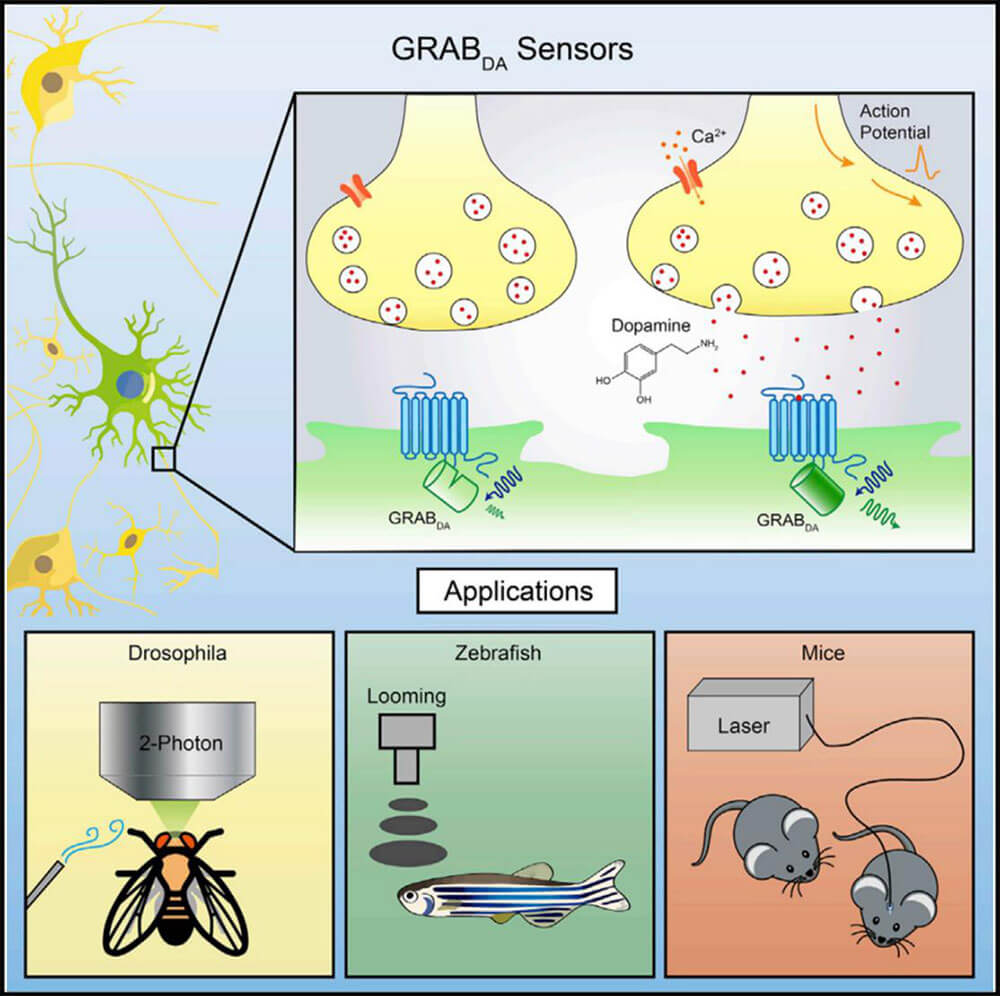
The conformational changes of the GPCR after binding to the ligand (neurotransmitter) are coupled to the changes in the fluorescence signal of the fluorescent protein, and the release of the neurotransmitter is detected using fluorescence imaging. There is a region in GPCR where the conformation changes most significantly after ligand binding. The cyclically rearranged fluorescent protein is inserted into this region to form a fusion protein. Once the release of a specific neurotransmitter activates the GPCR and causes it to undergo a conformational change, this conformational change will cause the fluorescent protein linked to it to also undergo a conformational change, ultimately changing its fluorescence intensity. Therefore, changes in the fluorescence intensity of fluorescent proteins can be used to indicate dynamic changes in the concentration of neurotransmitters. They named the fluorescent sensor GPCR Activation Based Sensor, or GRAB sensor for short.
At present, more than 10 new GRAB sensor (including dopamine, acetylcholine, norepinephrine, etc.) have been published in relevant literature. Such sensors can accurately detect dynamic changes in neurotransmitters in real time. Researchers can express sensors in a variety of cultured cells, mouse brain slices, or living Drosophila, zebrafish, mice and other model animals through transfection, virus injection, and construction of transgenic animals. Invisible and intangible chemical signals are transformed into intuitive and easily measurable fluorescent signals, which makes it easier to monitor dynamic changes in neurotransmitters. The emergence of new imaging sensors helps to understand changes in neurotransmitters in specific diseases, thus providing new paths for future precision medicine and new drug development.
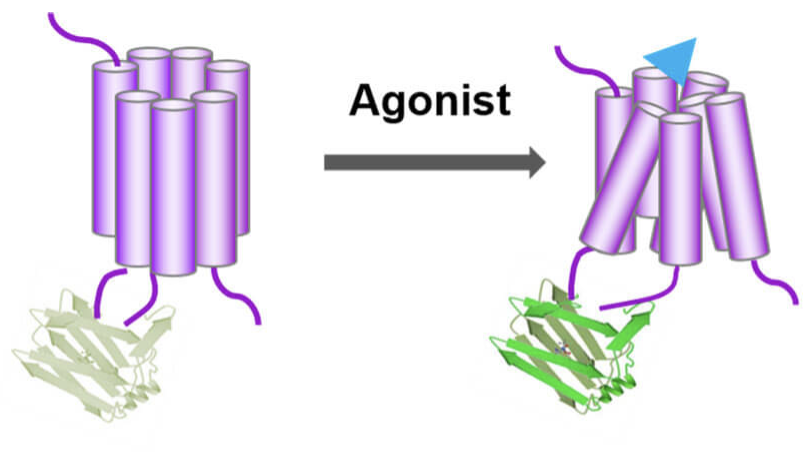
Schematic diagram illustrating the principle of neurotransmitter GRAB sensor
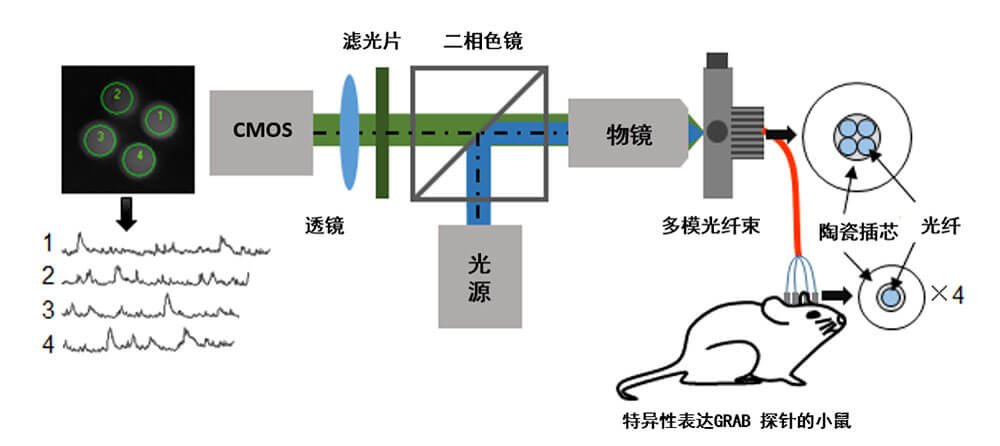
Experimental procedure: A multi-channel optical fiber recording system records the release of endogenous transmitters in freely moving mice
| Name | Function |
| DA | dopamine sensor |
| Ach | Acetylcholine sensor |
| NE | norepinephrine sensor |
| SHT | Serotonin sensor |
| Ado | Adenosine sensor |
| ATP | Adenosine triphosphate sensor |
| CCK | cholecystokinin sensor |
| VIP | Vasoactive intestinal peptide sensor |
| eCB | Endocannabinoid sensor |
Brain Case offers experimental services including optogenetic and chemogenetic manipulation, fiber photometry for calcium and neurotransmitter signal recording, EEG, EMG, and patch-clamp electrophysiology. These services are well-suited for studies of neural activity and function, as well as for evaluating neuropharmacological and neurotoxicological effects.