- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Brain Case' proprietary BRAVE high-volume and high-precision AAV serotype screening platform is capable of screening AAV serotypes with higher infectivity and higher tissue specificity for clinical trial studies, greatly reducing the dosage and production cost of AAV, and increasing safety and effectiveness.
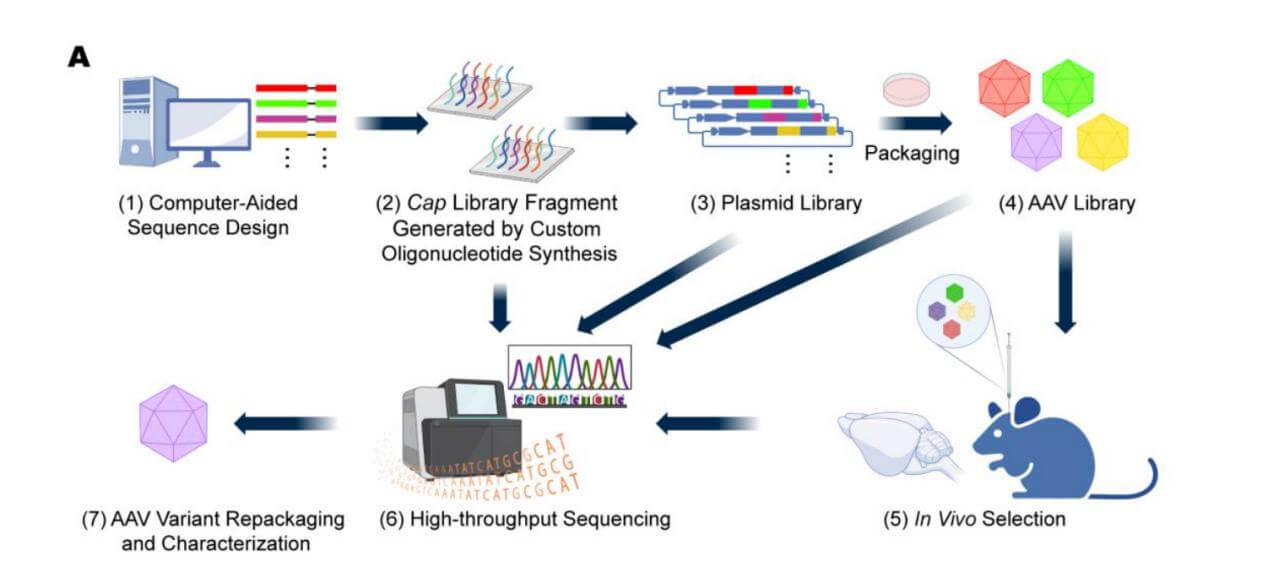
Figure1: Computer-aided AAV capsid design, virus library packaging, and in vivo screening process diagram. PMID: 37112829 PMCID: PMC10143561 DOI: 10.3390/v15040848
Today, the application of "artificial intelligence + biomedicine" in various subfields of biomedicine holds the potential to extensively reshape the current landscape of biomedical research and industry. We are dedicated to driving the development of AI technologies and platforms, leveraging our proprietary AAV high-yield system with large capacity and high-precision AAV mutagenesis engineering and screening platform. With a focus on "cost reduction and efficiency enhancement", we aim to develop AAV vectors that are more efficient and precise in targeting specific tissues or cell types, thus serving fields such as gene therapy and cell therapy.
Excitingly, through this technique, we have successfully screened AAV serotypes that can be widely applied in both research and clinical settings. Some of these serotypes have already been published in literature, please refer to the following link: