- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Vesicular stomatitis virus (VSV) is a non-pathogenic, enveloped, negative-sense RNA rhabdovirus that mainly infects rodents, cattle, pigs, and horses, etc. Low, only individual cases of infection among animal keepers and laboratory personnel. Symptoms are mild or asymptomatic after infection.
VSV can infect almost all types of cells, but cannot initiate productive infection in healthy cells due to type I interferons (Interferons, IFN)-mediated antiviral responses. However, defects in IFN signaling often coincide with tumorigenesis [2-3]. Therefore, VSV is able to infect and selectively destroy cancer cells with minimal damage to normal cells, and at the same time, the low anti-VSV immunity of the general population makes it an ideal oncolytic virus therapeutic agent [4].
The full length of the VSV genome is 11161nt, and it encodes five proteins sequentially from the 3' end to the 5' end: nucleocapsid protein (Nucleocapsid Protein, N), phosphoprotein (Phosphor Protein, P), matrix protein (Matrix Protein, M), sugar Protein (Glycol Protein, G) and large polymerase protein (Large Polymerase Protein, L) [5]. The G protein on its envelope initiates the infection process of the virus by binding to a variety of molecules widely present on the surface of the cell membrane [6]. M proteins have been shown to suppress innate antiviral responses and alter host transcriptional machinery, ultimately forcing tumor cells to undergo apoptosis [7]. The extensive cell tropism of VSV is based on the G protein. In order to improve the safety and selectivity of the oncolytic vector of VSV, it is usually replaced by membrane proteins of other viruses [8].
In 2000, Stojdl et al. [9] confirmed the potential of VSV as an oncolytic virus for the first time. They found that VSV could significantly inhibit the growth of melanoma in nude mice, but had little effect on normal tissue cells. Subsequently, the oncolytic properties of VSV were also confirmed in a variety of tumor models [10]. VSV has many advantages as an oncolytic virus:
Although VSV has many advantages in tumor therapy, wild-type VSV still has many defects, which is why it needs to be further modified and optimized. The first is the safety issue, mainly manifested in the potential neurotoxicity of VSV. A large number of studies have confirmed the intracranial administration of VSV in rodent models [12], nasal administration [13], intravascular administration [14] and Neuropathy caused by intraperitoneal administration [15], and severe neurotoxicity has also been observed in the thalamus of primates [16]; secondly, the short residence time of the virus in the body affects the oncolytic effect. Multiple administrations are often required in oncolytic therapy, but VSV-G protein can easily cause the body to develop adaptive immunity. When repeated administration, a large number of neutralizing antibodies against VSV in the body will cause VSV to be quickly cleared and fail to reach the goal. To achieve the ideal therapeutic effect, so the research at this stage is devoted to improving the anti-tumor activity and safety of rVSV.
Modification of key protein genes of VSV The neurotoxicity of wild-type VSV is mainly related to the cell-killing effect of its M protein and the neurotropism of its G protein. The modification of these two protein genes is the main direction to reduce the neurotoxicity of VSV. Studies have shown that deleting the 51st amino acid methionine on the VSV-M protein (VSVrM51) or replacing it with arginine (rM51R-M) can relieve the inhibitory effect of the M protein on the expression of interferon in infected cells, thereby Improve the safety of the virus in normal tissue cells [17-18].
Janelle et al[19] found that the G protein mutant virus strains of VSV (G5, G5R, G6 and G6R) can achieve similar effects to the M protein mutation (rM51R-M), that is, the expression of interferon is increased and the neurotoxicity is significantly weakened; Roberts [20] reduced the replication ability of VSV in the nervous system by truncating the G protein from the 29th amino acid to the ninth amino acid (CT9) or the first amino acid (CT1); Duntsch et al. [21] In the study, the recombinant VSV vector (rVSV-rG) with the deleted G protein gene was used to package virus particles with single cycle replication in cells transiently expressing VSV-G. The killing effect of glioma was no less than that of wild-type VSV.
During the process of infecting and killing tumor cells, oncolytic viruses will also cause the body's immune response to the virus itself. Circulating antibodies in the body, non-specific host proteins or complement proteins neutralize virus particles, which will cause them to be quickly eliminated. Clearance will ultimately affect the effect of oncolytic therapy.
At present, there are many strategies, the first is to use cell carrier delivery, aptamer technology, PEG covalent modification, etc., to temporarily isolate VSV before reaching the tumor site, thereby reducing the contact between VSV and neutralizing antibodies in the blood circulation and being non-specific. Neutralization of host proteins or complement proteins to prevent the virus from being cleared prematurely by the immune system. Tesfay et al. [22] confirmed that PEGylation of VSV effectively prolongs the half-life of the virus in mice by passive immunization with VSV neutralizing antibodies. The second strategy is to make it more conducive to the replication of VSV by regulating the immune microenvironment in vivo. Muik et al. [23] used chimeric virus to replace VSV-G with LCMV-G, which is not easy to induce neutralizing antibody production. The chimeric virus formed can effectively reduce the production of neutralizing antibody; VSV prepared by Altomonte et al. [24] Recombinant virus VSV-gG can secrete a broad-spectrum chemokine-binding protein, equine herpesvirus type 1 glycoprotein (gGEHV-1), and the introduction of gGEHV-1 can significantly reduce tumor in a rat model of hepatocellular carcinoma NK cells and NKT cells in the microenvironment can greatly increase the virus titer in the tumor and thus improve the therapeutic effect; similarly, Wu et al[25] proved that the recombinant virus VSV (MΔ51)- M3 for the treatment of multifocal hepatocellular carcinoma in rats can reduce the accumulation of NK cells and neutrophils in the lesion, thereby increasing the virus titer and oncolytic effect in the tumor.
To enhance the direct killing effect of VSV on tumor tissue, currently proven effective methods include integrating tumor suppressor genes, suicide genes, enhancing the infiltration effect of VSV on tumor tissue, and combining other treatment methods.
(1) Integrate tumor suppressor genes. p53 is an important tumor suppressor gene in the body. It can effectively inhibit the occurrence and development of tumors by regulating cell cycle signaling pathways to mediate DNA repair, cell senescence, apoptosis, and immune activation [26], and has been used in a variety of oncolytic virus combined therapy.
(2) Integration of suicide genes. The integration of the suicide gene can not only enhance the lethality of VSV to infected tumor cells, but also enable VSV to kill surrounding uninfected tumor cells through the gap junction between cells, which is a very effective way to enhance the lethality of tumor cells. effective strategy. TK/GCV is a suicide gene system widely used in the field of gene therapy. The non-toxic prodrug ganciclovir (GCV) can be phosphorylated by herpes simplex virus thymonic kinase (HSV-TK) to its diphosphate and Three: Phosphate metabolites, which mediate tumor cell apoptosis by inhibiting host cell DNA polymerase activity, and effectively induce the activation of tumor-specific killer T cells [27].
Enhance the infiltration ability of VSV in tumor tissue. Ebert et al[28] inserted the fusion protein (NDV/F) mutant L289A gene of Newcastle disease virus (NDV) into the VSV genome, and the constructed recombinant VSV could induce tumor cells to grow under neutral pH conditions. Membrane fusion of the drug induces the formation of syncytia, thereby accelerating the spread of the virus in the tumor, effectively improving its killing ability against human and mouse hepatocellular carcinoma, and animal experiments have also shown good therapeutic effects.
In addition to enhancing the direct killing effect on tumors, the induction of stronger tumor-specific immune responses by VSV is also crucial for the complete cure of tumors.
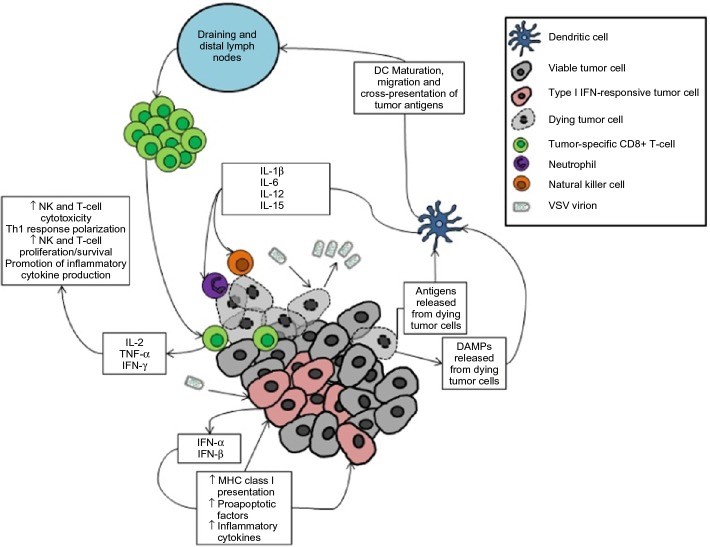
Figure 2: VSV oncolytic virotherapy can generate anti-tumor immunity due to the viral infection of transformed cells(DOI: 10.2147/OV.S66079).
Note: Tumor reduction is achieved through both direct and indirect mechanisms. Viral replication within tumor cells leads to cytopathic effects and ultimately to the death of infected cells. Likewise, the innate antiviral response of infected cells results in tumor cell death through downstream effects of type I interferon signaling. This is combined with the release of tumor antigens and danger-associated molecular patterns (DAMPs), stimulating the maturation of local dendritic cells. The mature dendritic cells secrete pro-inflammatory cytokines to recruit innate effector cells to mediate tumor killing, and they migrate to tumor-draining and distant lymph nodes to present tumor antigens to tumor-reactive CD8+ T cells. The activated T cells then migrate to the tumor and mediate antigen-specific attacks on tumor cells.
In recent years, with the continuous development of the VSV oncolytic virus platform, many oncolytic virus recombinant variants based on VSV transformation have emerged, mainly aimed at improving tumor targeting, prolonging the retention time in vivo, and enhancing oncolytic effects. The safety and effectiveness of VSV oncolytic virus for tumor treatment have been improved, and a series of VSV-based oncolytic virus preparations have gradually entered the clinical trial stage.
| # | Vector type | Indications | Clinical summary |
|---|---|---|---|
| 1 | VSV-hIFNB-NIS | Solid tumors non-small cell lung cancer neuroendocrine tumors |
The aim of this study is to determine the optimal dosage of VSV-IFNB-NIS in combination with pembrolizumab in patients with solid tumors |
| 2 | VSV-IFNbetaTYRP1 | Thornton | The study investigated the safety and optimal dosage of the modified virus VSYIPNbeaTYRP in treating patients with stage III-IV melanoma. Two genes were modified in the vesicular stomatitis virus (VSV): human interferon B (hIFNbeta), which can protect normal healthy cells from viral infection; and TYRP1, which is primarily expressed in melanocytes and melanoma tumor cells, potentially triggering a strong immune response to kill melanoma tumor cells. |
| 3 | VSV-GP | Colon cancer rectal cancer | vSV-GP is a recombinant chimeric vesicular stomatitis virus carrying the envelope glycoprotein (GP) of the visceral non-neurotropic WE-HIPI strain of lymphocytic choriomeningitis virus (LCMV, Arenaviridae) instead of the native vSV glycoprotein (G). |
| 4 | VSV-hIFNB-NIS | Malignant solid tumors | The study consists of three parts: a single-agent dose-escalation phase of ITVSV-IFNB-NIS monotherapy, a selection phase of single-agent therapy intravenous regimens, and an expansion phase aimed at exploring the safety, efficacy, MTD, PK, and tumor and biomarker responses of the selected single treatment alone or in combination with avclumab for patients with metastatic colorectal cancer |
| 5 | VSV-hIFNB-NIS | Endometrial cancer | This phase trial investigated the adverse effects and optimal dose of Vesicular stomatitis virus-human interferon beta-NIS (VSV-hIFNB-NIS) with or without transporter in combination with or without phospho-lutetinib for the treatment of stage IV or recurrent endometrial cancer patients. VSV-hIFNB-NIS limits its ability to spread to tumor cells. It also contains a gene for a protein called NIS, which helps the body concentrate iodine, allowing tracking of the virus's whereabouts. VSV-hIFNB-NIS may be able to kill tumor cells without harming normal cells |
| 6 | VSV-hIFNB-NIS | Multiple myeloma lymphoma | This phase trial investigated the optimal dose and adverse effects of recombinant vesicular stomatitis virus carrying human NIS and IFNB genes (VSV-hIFNB-sodium iodide symporter NIS) in combination with or without phospholipase-tyrosine kinase inhibitor for the treatment of multiple myeloma, acute myeloid leukemia, or T-cell lymphoma patients. VSV-hIFNB-NIS may be able to kill cancer cells without harming normal cells. Phospho-lutetinib can inhibit the growth of tumor cells by blocking some enzymes needed for cancer cell growth. Combining VSV-hIFNB-NIS with phospho-lutetinib may provide better treatment for multiple myeloma, acute myeloid leukemia, and T-cell lymphoma |
Table 1: Clinical-stage vesicular stomatitis virus oncolytic virotherapy
VSV has achieved certain results in the field of oncolytic viruses and has a very broad application prospect. VSV also exhibits various advantages as a therapeutic platform, such as high immunogenicity, relatively easy transgene insertion into its genome, easy management, and extensive tropism However, in order to achieve tumor cure and even prevent recurrence, more effective treatment strategies need to be explored. Therefore, in addition to obtaining recombinant VSV strains with better therapeutic effects through transformation, exploring the combination of recombinant VSV oncolytic virus with immune checkpoint inhibitors, targeted therapy drugs and other therapeutic methods is also a research method with development potential.
| Product No. | Product Name | Illustration |
| BC-VSV-Vs03 | VSV-EYFP | anterograde, Green |
| BC-VSV-Vs13 | VSV-mCherry | anterograde, Red |
| BC-VSV-Vs28 | VSV-WT | Wild type, No color |
| BC-VSV-Vs31 | VSV-ΔG-EYFP | Transformable pseudoviruses and oncolytic viruses |
| BC-VSV-Vs33 | VSV-ΔG | Functional components can be added and Transformable pseudoviruses and oncolytic viruses |
literature citation