- E-mail:BD@ebraincase.com
- Tel:+8618971215294
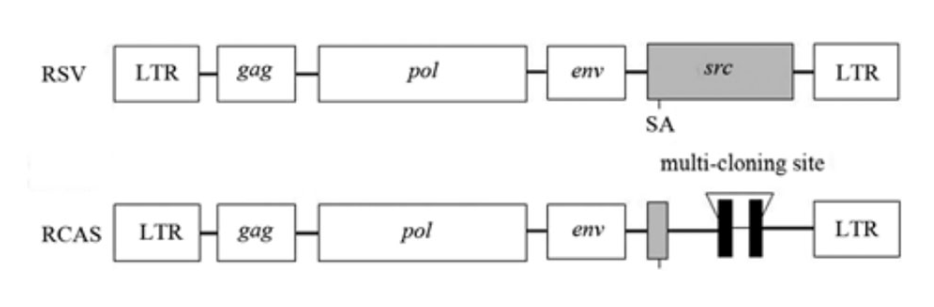
RCAS virus is a member of the retrovirus family derived from the SR-A strain Rous sarcoma virus (RSV), a replicating avian sarcoma-leukemia virus (ASLV) with splicing receptors long terminal repeat (LTR). The src oncogene of RSV was deleted and a polyclonal site was inserted that could stably accommodate up to 2.5kb of inserted fragments (Figure 1). The expression of the inserted gene may be driven by the viral long terminal repeat (LTR) or by an appropriate internal promoter.
The RCAS-TVA gene delivery system is based on the RCAS virus specifically recognizing the avian sarcoma leukemia virus subgroup A(ASLV-A) receptor (TVA) to enter and infect cells. TVA is a member of the low-density lipoprotein receptor family, encoded by the tv-a gene. The mRNA transcribed by the tv-a gene is selectively spliced to produce at least two proteins, a transmembrane protein and a GPI-anchored isoform, both recognized by ASLV-A. Mammalian cells lack the gene that codes for TVA and are generally resistant to ASLV-A or RCAS virus infection; However, targeting TVA transgenes to specific cell types or mouse tissues can make these cells vulnerable to ASLV-A based RCAS virus infection. RCAS viruses retain all the viral genes needed for replication, and when the RCAS vector enters the cell, it can directly encode the infectious particles and assemble them into the desired proteins, so no helper cells are needed. High titer RCAS virus can be obtained by transfecting RCAS vector directly into avian cells, such as DF1 cells. The generation process based on RCAS-TVA model is shown in Figure 2 [1]. The expression of specific proto-oncogenes by RCAS can induce specific types of cells or mouse tissues to become cancerous and form tumors.
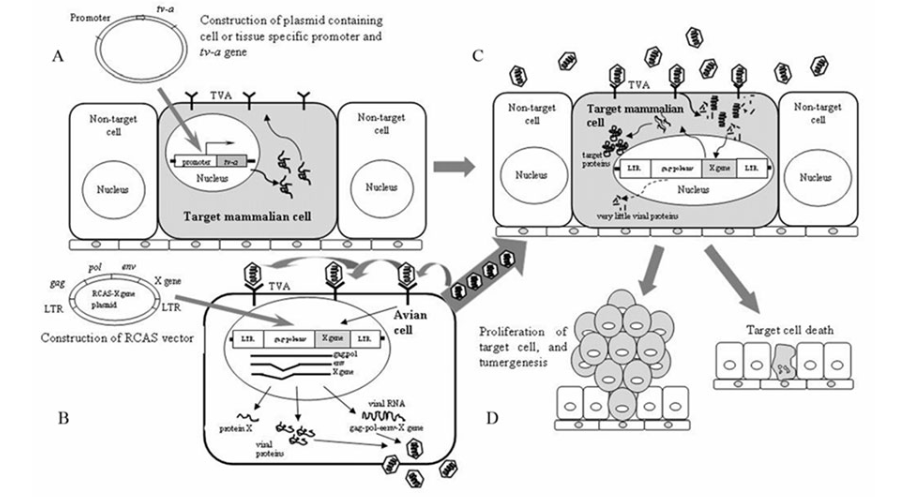
Fig.2 Schematic drawing ofthe RCAS-TVA technique in mammalian system
The RCAS-TVA system provides a simple and rapid way to study gene function in mouse models of human cancer. These models provide important insights into the cells of origin of specific tumors and the role of initial genetic lesions in determining tumor types. The RCAS-TVA model can be combined with existing transgenic, knock-out or knock-in models, providing opportunities to extend the utility of these models.
Reference
1)Ahronian L G, Lewis B C. Using the RCAS-TVA system to model human cancer in mice[J]. Cold Spring Harbor Protocols, 2014, 2014(11): pdb. top069831.
2)Lewis B C, Klimstra D S, Varmus H E. The c-myc and PyMT oncogenes induce different tumor types in a somatic mouse model for pancreatic cancer[J]. Genes & development, 2003, 17(24): 3127-3138.
3)Holloway K R, Sinha V C, Toneff M J, et al. Krt6a-positive mammary epithelial progenitors are not at increased vulnerability to tumorigenesis initiated by ErbB2[J]. PLoS One, 2015, 10(1): e0117239.
4)Haricharan S, Hein S M, Dong J, et al. Contribution of an alveolar cell of origin to the high-grade malignant phenotype of pregnancy-associated breast cancer[J]. Oncogene, 2014, 33(50): 5729-5739.
5)Lewis B C, Chinnasamy N, Morgan R A, et al. Development of an avian leukosis-sarcoma virus subgroup A pseudotyped lentiviral vector[J]. Journal of virology, 2001, 75(19): 9339-9344.