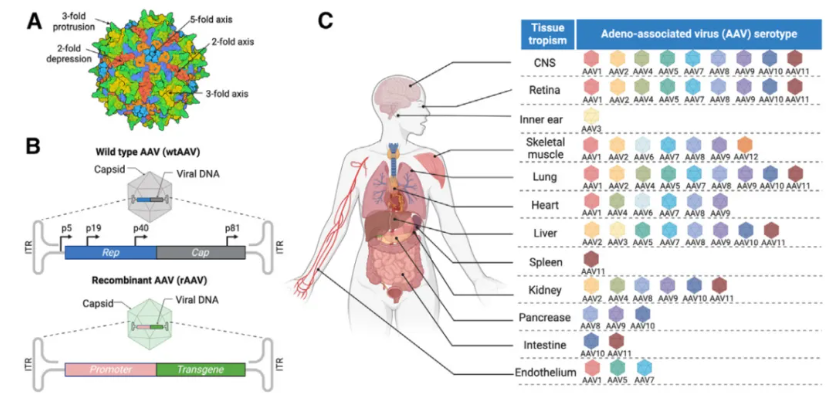
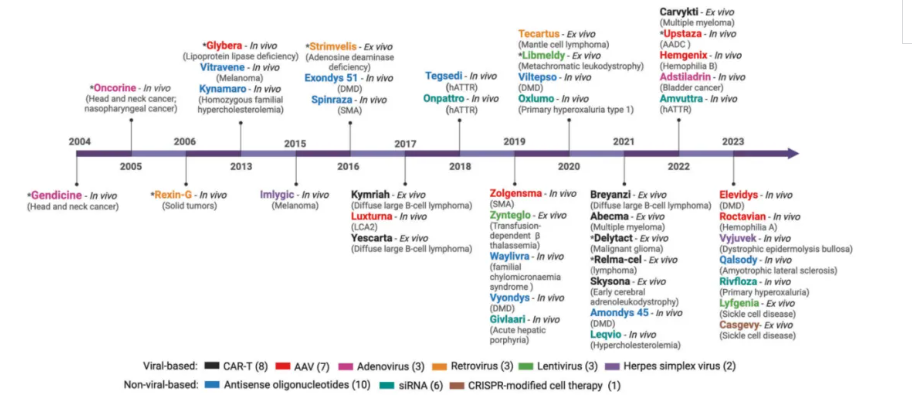
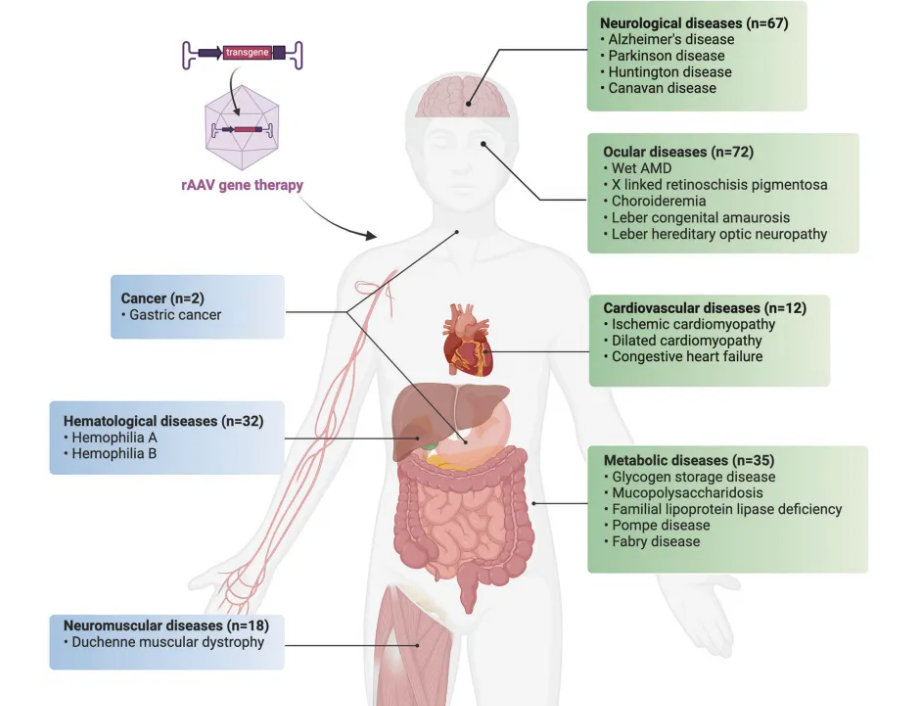
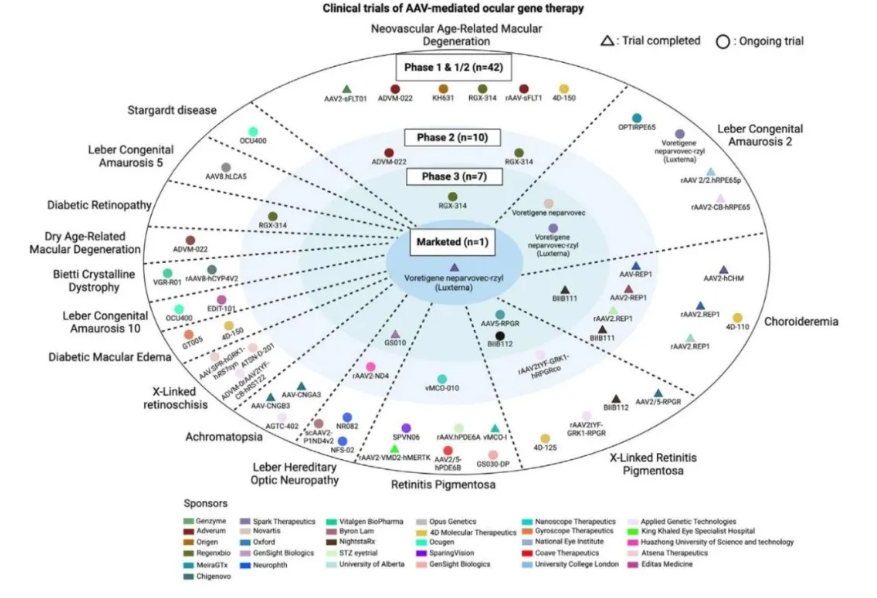
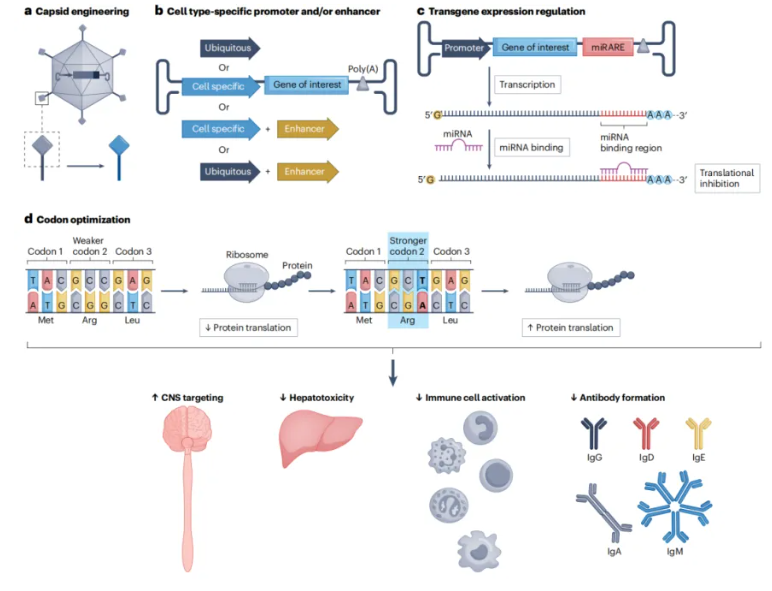
🎗Aromatic L-amino acid decarboxylase deficiency (AADCD)
rAAV2.AADC is a gene therapy strategy for the treatment of AADCD. AADCD is an autosomal recessive disorder caused by mutations in the dopa decarboxylase gene that results in serotonin and dopamine deficiencies. In 2012, four children with AADCD in Taiwan were treated with bilateral lenticular injection of rAAV2.AADC (1.6 × 10^11 vg), and all patients were found to have improved motor scores and subjective emotional stability. In a follow-up phase I/II trial of 10 patients, a slightly higher dose (1.81 × 10^11 vg) was used, and all patients experienced improved clinical motor function and cerebrospinal fluid (CSF) biochemistry. However, the patient developed transient dyskinesia after the infusion.
Recently, long-term follow-up results from the Taiwan trial reported significant improvement in 26 patients, three of whom were able to walk, and found that treatment age was inversely associated with exercise scores, but not with dose and scores. The most common treatment-emergent side effects (TEAEs) are fever and dyskinesia. Movement impairment was relieved in all but 1 patient, and the severity and duration of movement impairment were positively correlated with treatment age. The therapy (Eladocagene Exuparvovec) was finally licensed and approved by the European Medicines Agency for patients over 18 months of age (Upstaza).
🎗Spinal muscular atrophy (SMA)
Onasemnogene abeparvovec (Zolgensma) is a gene therapy used to treat SMA. SMA is an autosomal recessive disorder caused by loss-of-function mutations in the SMN1 gene. SMA type I is the most severe type, usually presenting within 6 months of life, and patients often die before the age of 2 years. In 2017, the results of the first clinical trials were announced, in which 15 patients received intravenous rAAV9-expressing SMN1 (rAAV9.SMN1) gene therapy, which showed a reduced need for ventilator support and an increase in exercise scores. Eleven patients were able to sit and stand for several seconds without the aid of external force and gained speech skills. Although the therapy is well tolerated, a common adverse effect is elevated hepatic transaminases, which are relieved with corticosteroids. Long-term follow-up results from the START trial, published in 2021, showed that patients maintained the motor skills acquired during the initial trial after a maximum follow-up of 6.2 years. Even more encouragingly, two patients reached new motor development milestones and were able to stand with assistance. In terms of safety, the results remained very positive, with no treatment-related adverse effects (TEAEs) reported.
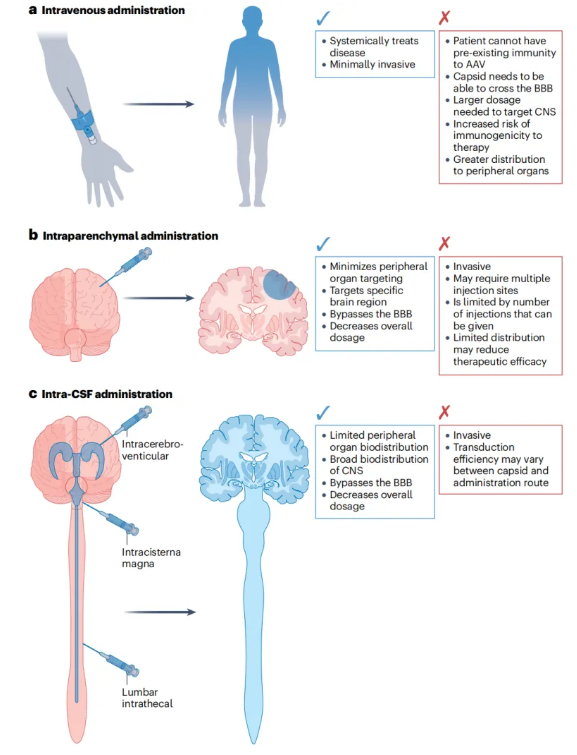
Figure 5: Route of administration of CNS gene therapy
3. Metabolic diseases
Genetic defects leading to metabolic disease may have systemic or organ-specific manifestations that have implications for vehicle design and route of administration (e.g., lysosomal storage disorders with central nervous system predominantly manifestations).
🎗Tate-Sachs Disease (TSD) and Sandhoff Disease (SD)
rAAV.rh8 is a gene therapy strategy for the treatment of TSD and SD. TSD and SD are caused by mutations in the HEXA gene, which is a dimer composed of α and β (HEXA), β and β (HEXB), or α and α (HEXS) subunits, which result in ganglioside accumulation, and TSD and SD are caused by mutations in genes encoding α and β subunits, respectively. In 2022, an extension use trial in 2 patients with TSD demonstrated that the rAAV.rh8 vector expressed by intrathecal injection of two α and β subunits was safe and well tolerated by the procedure and vector, with both patients showing signs of stable disease. Currently, two ongoing clinical trials in Canada and the U.S. (project numbers NCT04798235 and NCT04669535, respectively) are evaluating the efficacy of rAAV9. The Phase I/II trial in Canada utilizes rAAV9 to deliver HEXA and HEXB transgenes to patients with infantile TSD or SD through a single intrathecal dose. In contrast, the U.S. trial was designed as a dose-escalation study, exploring four different vehicle doses through a bilateral thalamic route and intrathecal/cisternal combination administration. The study used two rAAV.rh8 vectors to deliver HEXA or HEXB transgenes, respectively, and the results of these trials have not yet been published.
🎗Canavan Disease (CD)
CD is caused by mutations in the ASPA gene, which leads to the accumulation of N-acetyl aspartate (NAA) in the central nervous system and urine. Currently, there are two clinical trials underway for CD, with trial numbers NCT04833907 and NCT04998396. The two trials used different vehicle designs and modes of administration (intracranial versus intravenous infusion), and different routes of administration inevitably led to differences in doses. Preliminary data from two trials show a positive treatment effect. As the two trials are still ongoing, a direct comparison of effects is not possible at this time.
4. Hematologic diseases
Non-neoplastic hematologic disorders can be classified as cellular or non-cellular, meaning that genetic mutations affect cellular function (e.g., sickle cell disease) or blood components (e.g., hemophilia), respectively. This distinction is crucial for gene therapy because it determines the target cell/organ.
🎗Hemophilia B
rAAV5 Padua (Hemgenix) is a gene therapy drug used to treat hemophilia B. HEMOPHILIA B IS CAUSED BY A DEFICIENCY OF COAGULATION FACTOR IX. (FIX.), WHICH IS PRODUCED IN LIVER CELLS. In 2009, researchers discovered the Padua variant of FIX, which has supraphysiological activity. In a phase I clinical trial, patients received a combination of the intravenously administered hepato-stimulating drug rAAV8 and an optimized codon hFIX transgene with FIX activity of 2-12% and no immunosuppression. In a subsequent study, the researchers designed a new engineered capsid with enhanced hepatropy, combined with the codon-optimized Padua FIX variant, with a corresponding dose reduction, and the FIX activity still reached an average of 33.7% (range 14-81%). In addition, only 2 patients were treated with prednisolone for transient elevated liver enzymes, showing a favorable safety profile. Based on these results, Hemgenix received FDA approval in 2023, becoming the first gene therapy approved for the treatment of hemophilia B.
🎗Hemophilia A
rAAV5hFVIIISQ (Roctavian) is a gene therapy drug used to treat hemophilia A. Hemophilia A is caused by a deficiency of coagulation factor VIII (FVIII), which is synthesized in endothelial cells. In a phase III clinical trial, patients received a single intravenous dose of rAAV5hFVIIISQ. The results showed that this one-time treatment significantly increased FVIII activity, reduced exogenous FVIII use, and improved bleeding events compared to routine prophylactic use of exogenous FVIII. However, over time, transgene expression declined. Despite this, Roctavian received FDA approval in 2023, becoming the first gene therapy approved for the treatment of hemophilia A.
5. Neuromuscular diseases
Neuromuscular diseases include diseases that affect neurons and muscle cells, with muscular dystrophy mainly affecting muscle cells. Duchenne muscular dystrophy (DMD) is the most common form of muscular dystrophy and is caused by mutations in the dystrophin gene that results in dystrophin deficiency or dysfunction.
rAAV.rh74.micro-dystrophin (Elevidys) is a gene therapy drug used to treat Duchenne muscular dystrophy (DMD). In 2020, a phase I/II trial evaluated the safety and efficacy of rAAV.rh74.micro-dystrophin administered intravenously at 2.0 × 10^14 vg/kg. The results showed improvement in clinical and histopathological manifestations in all patients, and correlated with age and disease severity at the time of treatment. In 2023, Elevidys received FDA approval, becoming the first gene therapy approved for the treatment of DMD.
In addition to gene replacement therapy, another treatment strategy is the use of disease-modifying genes. In 2022, a first-in-human trial treated two patients (6.9 and 8.9 years). In the experiment, bilateral femoral veins were used to isolate limb perfusion, and 5.0 × 10^13 and 2.5 × 10^13 vg/kg of rAAV.rh47 were injected into each leg to express the GALGT2 gene. No associated hepatotoxicity was reported, although antibodies against the rAAV capsid and mild rAAV and GALGT2 protein-specific T cell activity were detected. Notably, younger patients who received higher doses showed greater improvement. Other ongoing trials are still ongoing but are not recruiting new patients, including the randomized Phase I/II trial (NCT03368742) at Solid Bioscience and the non-randomized Phase Ib trial (NCT03362502) at Pfizer, both of which utilize rAAV9 to express truncated dystrophin under the control of a muscle-specific promoter.
6. Cardiovascular disease
Heart failure (HF) is a syndrome caused by dysfunction of ventricular filling or emptying, and preclinical studies have shown that an effective strategy is to overexpress sarcoplasmic/endoplasmic reticulum calcium transport ATPase 2 (SERCA2a), which pumps calcium into the sarcoplasmic reticulum (SR) during diastole, thereby improving cardiac function.
In a phase I/II CUBIC trial, the effect of overexpressing SERCA2a with rAAV1 (rAAV1.SERCA2a) was evaluated. The results of the phase I study showed that some patients had improved symptoms and were well tolerated to treatment. In a further phase II study, a total of 25 treated and 14 placebo patients were included, and the treated patients showed a trend of improvement compared to those who received placebo; However, with the exception of left ventricular end-diastolic volume, most of the results did not reach a statistical difference, and the study confirmed the good safety profile observed in stage I. Subsequent three-year follow-up of CUPID phase II also failed to show an improvement in survival, but a statistically significant difference was reached in the cumulative analysis of non-terminal events.
7. Cancer
rAAV anti-tumor strategies are similar to conventional anti-tumor regimens, with gene therapy offering specific advantages, such as regulation or knockdown of gene expression. rAAV can be transduced into dividing and non-dividing cells, enabling broad cell targeting in heterogeneous tumor tissues. This property can be used to deliver cytokine transgenes to tumor cells to recruit immune cells to trigger anti-tumor immune responses.
The use of rAAV in humans is relatively limited, and only one trial (NCT02496273) of in vitro gene therapy for gastric cancer is currently ongoing. In a proof-of-concept study, rAAV-mediated interferon β (IFNβ) expression was used to inhibit the growth of colorectal cancer cells in a xenograft mouse model. The adaptability of rAAV can also improve transduction efficiency and specificity and mitigate adverse effects. For example, rAAV with a Her2 ligand increases transduction specificity. Expression of HSV thymidine kinase converts ganciclovir into a toxic product that is subsequently integrated into tumor DNA to induce cell death. This delivery modality reduced tumor burden and prolonged survival compared to the group of animals that received the anti-cancer drug Herceptin. However, this method is time-consuming and labor-intensive. Another approach is to screen the existing rAAV capsid repertoire and determine the best combination based on tissue affinity in different routes of administration. For example, rAAV7 and rAAV9 have been found to be highly efficient in transducing into mouse prostate tissue.
🧠Challenges and solutions for AAV gene therapy🧠
1. Immune response
One of the biggest challenges in AAV gene therapy is the immune response. Pre-existing neutralizing antibodies (NAbs) can significantly reduce the transduction efficiency of rAAV, limiting the possibility of repeat dosing.
For example, in clinical trials at Luxturna and Zolgensma, some patients failed to achieve the desired response due to pre-existing NAbs. In addition, complement activation, innate immunity, and adaptive immune responses can also trigger adverse effects. For example, in Zolgensma's clinical trial, some patients developed thrombotic microangiopathy (TMA). To address these issues, researchers are exploring a variety of strategies, including the use of immunosuppressive drugs and the design of engineered capsids. For example, the use of drugs such as steroids, rituximab, and rapamycin can effectively suppress the immune response. In addition, optimizing the AAV capsid through computer-aided design and machine learning can improve transduction efficiency and tissue specificity, thereby reducing the immune response.
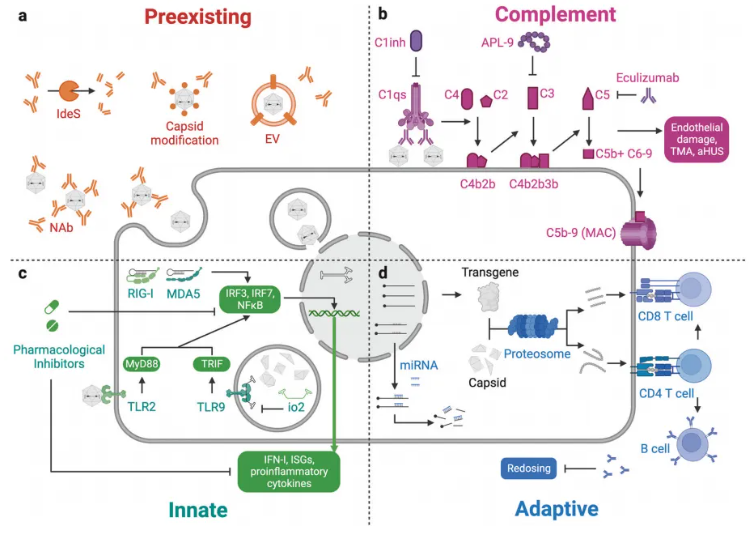
Figure 6: Immune response in rAAV-based gene therapy
2. Long-term efficacy and safety
The long-term efficacy and safety of AAV gene therapy remains a key issue. For example, the persistence of transgene expression, the potential risk of genome integration, and adverse effects such as hepatotoxicity and neurotoxicity all require further investigation. In addition, high-dose AAV treatment may trigger serious adverse reactions such as thrombotic microangiopathy (TMA). For example, in Zolgensma's clinical trial, some patients developed TMA. To address these issues, researchers are optimizing vector design, reducing the risk of genomic integration, and assessing safety through long-term follow-up studies.