- E-mail:BD@ebraincase.com
- Tel:+8618971215294
- Home
-
Virus product library
-
Products & Service
Product Center
Virus
- VSV-circuit research-vaccine and gene therapy research-BrainCase
- Retrovirus-RCAS-TVA-BrainCase
- Lentivirus Vector - Lentivirus Production - BrainCase
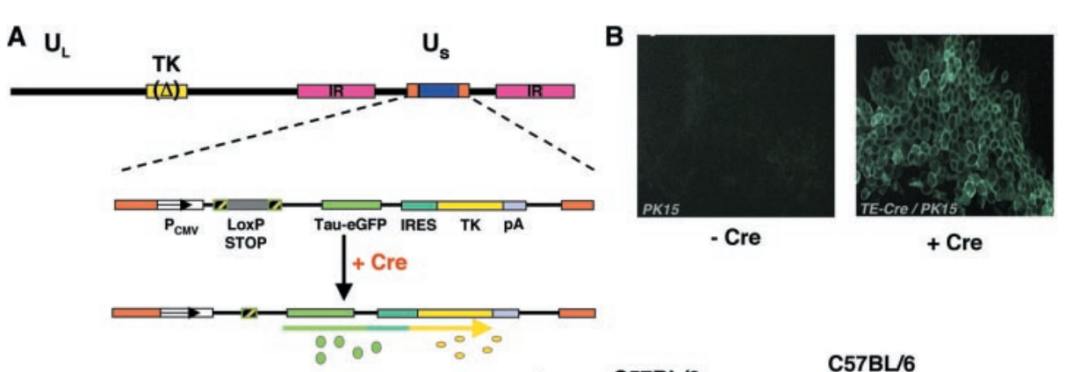
- Rabies Virus Vector - RBV Vector - BrainCase
- Herpes simplex virus-Oncolytic and anterograde tracing-Brain Case
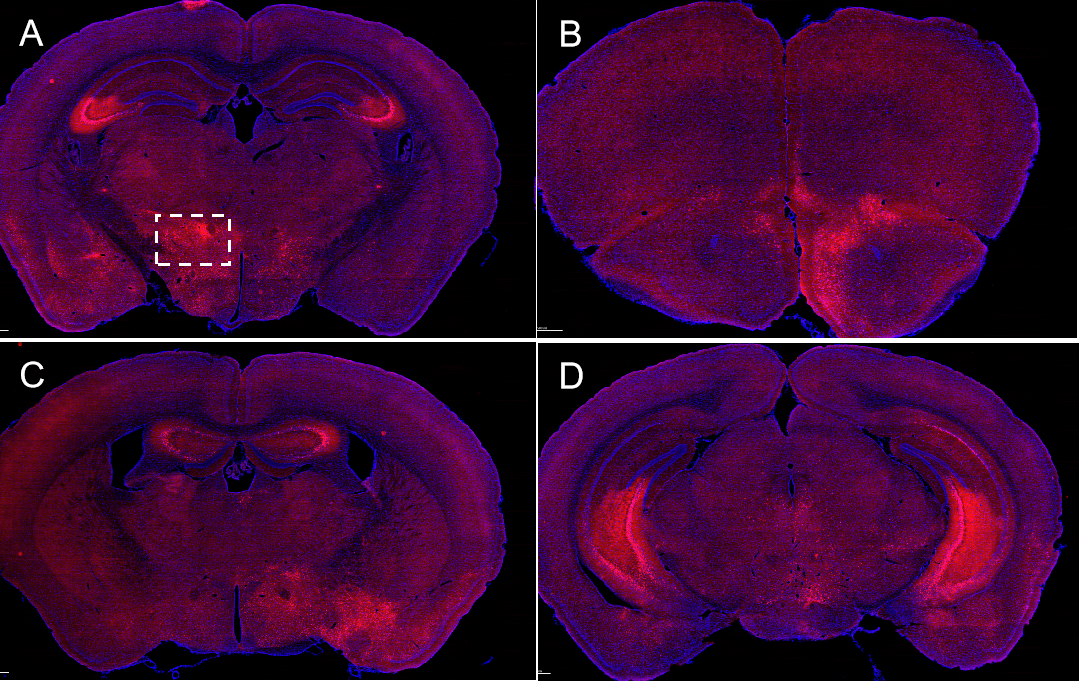
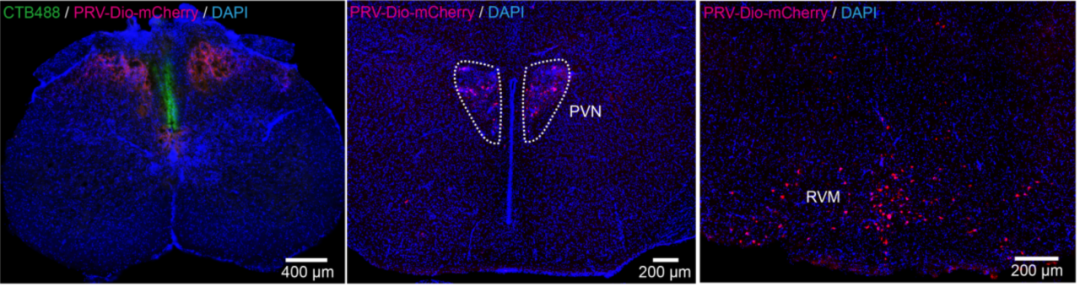
- PRV-retrograd multisynaptic-Peripheral-Btain Case
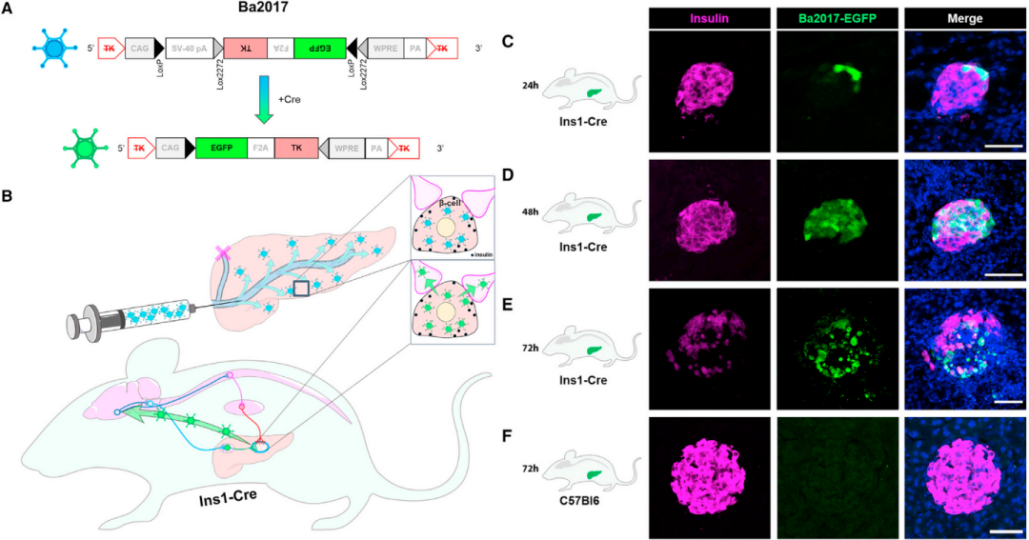
- AAV-gene therapy vectors-BrainCase
Popular Applications
-
News
-
Support
-
About Us
-
Contact
-