- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Neuroanatomical tracing techniques are fundamental to unraveling the complex network of brain connectomes. Current tools for tracing tracers that propagate between neurons, especially strictly anterograde transmonosynaptic tracers, are still lacking. The mutant H129-ΔTK of HSV1 strain H129 has been used as an anterograde transmonosynaptic tracer, however H129 has a severe defect in that it can infect neurons through axon terminals. Therefore, when using H129 to analyze output neural circuits, the interference caused by its reverse infection should be carefully considered. It is important to eliminate H129 axon terminal uptake and achieve strict anterograde transmonosynaptic tracing of H129.
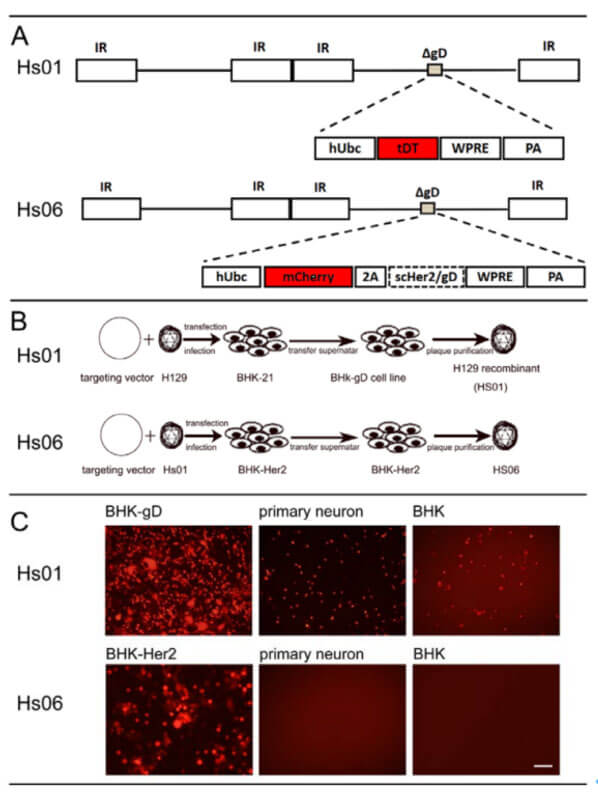
Xu Fuqiang's research group at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences reported a rigorous and practical anterograde transmonosynaptic tracer based on recombinant virus H129, which carries anti-human epidermal growth resistance in envelope glycoprotein D (gD) Single-chain antibodies (scFv) to factor receptor 2 (Her2) target specific types of neurons. By using helper viruses that complementally express Her2 and gD, direct projection network tracking of specific brain regions and neuron types can be achieved. Relevant work was published in the article "Rigorous anterograde trans-monosynaptic tracing of genetically defined neurons with retargeted HSV1 H129" on bioRxiv.
The author first co-transfected the constructed shuttle vector pHs01 with the H129 virus into the BHK cell line to obtain the gD-deleted H129 strain Hs01 virus (H129ΔgD-tdTomato); further, the Hs01 virus was co-transfected with the vector pHs06 (phUbc-mCherry-T2A-scHer2/gD- WPRE-pA) was co-transfected into the BHK-Her2 cell line to obtain Hs06 virus (H129ΔgD-hUbC-mCherry-P2A-scHer2::gd-WPRE-bGH polyA). Hs06 has the specificity to target the Her2 receptor, and cell infection experiments have shown that Hs06 can also specifically recognize the shortened Her2 receptor (Her2CT9). Since neurons in the central nervous system of animals hardly express Her2 receptors, the ability of H129 to infect axon terminals is eliminated.
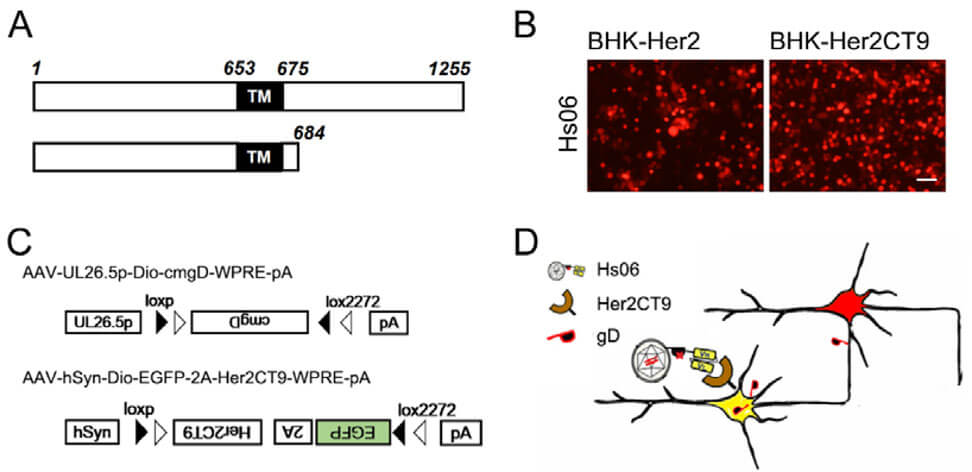
How does Hs06 infect the next level of neurons across synapses after entering the initial neuron? By designing the Hs06 helper virus AAV-UL26.5p-DIO-cmgD-WPRE-pA and compensating the codon-optimized gD protein (cmgD) in neurons, the Hs06 virus can be spread across synapses. At the same time, a helper virus carrying the Her2CT9 element was designed to achieve specific infection with Hs06 virus. The two helper viruses were injected into the target brain area in advance, and after the expression levels of Her2CT9 and EGFP were sufficient, the Hs06 virus was injected into the same location again. Hs06 enters neurons by recognizing Her2CT9, and then uses the gD expression element provided by the helper virus to complete the packaging of progeny, achieving anterograde trans-synaptic propagation.
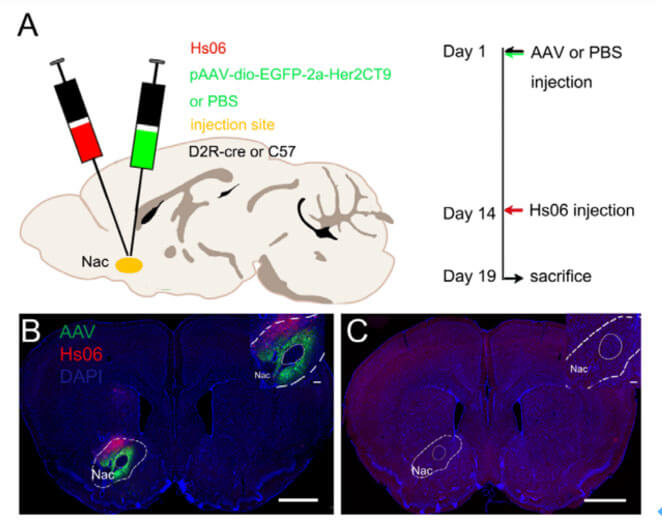
Is Hs06 still infection-specific in vivo? The author injected PBS and the helper virus AAV-hSyn-DIO-EGFP-T2A-Her2CT9 into the nucleus accumbens (Nac) brain area of D2R-Cre or C57BL/6 mice respectively. Two weeks later, Hs06 was injected in situ. Five days later, the brain tissue was collected. Section for observation. The results showed that a large number of Hs06-infected neurons could be seen in the Nac and adjacent areas of the brains of mice in the experimental group, while no red neurons were observed in the control group (Figure 3c). Therefore, it is further confirmed that Hs06 has a high affinity with Her2CT9, and the Hs06 virus can efficiently and specifically infect neurons expressing Her2CT9 in vivo.
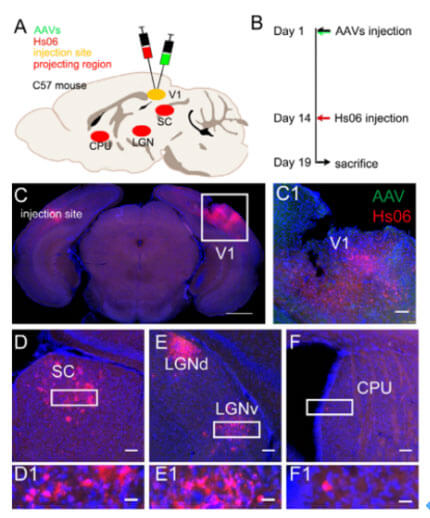
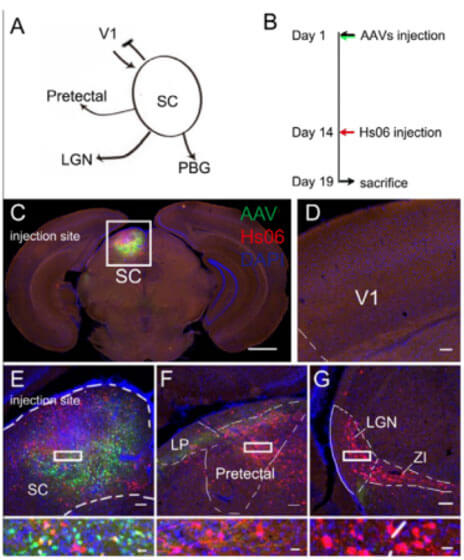
To further verify the anterograde propagation ability of Hs06. The authors conducted tracing experiments in the primary visual cortex (V1) of the mouse brain. First, the virus AAV-UL26.5p-Dio-cmgD and AAV-hSyn-Cre-WPRE-pA were mixed and injected into C57BL/6 mouse V1 at the same time as the helper virus AAV-hSyn-Dio-EGFP-T2A-Her2CT9. area, and 14 days later, Hs06 was injected into the same site. Five days after Hs06 injection, brain slices were taken for observation. Since the fluorescence expression level of Hs06 is low, the authors performed immunohistochemistry on the brain tissue to further amplify the fluorescence signal for imaging and observation. In the labeling results, red fluorescent protein can be observed in brain areas such as the superior colliculus (SC), lateral geniculate ventral area (LGNv), and caudate putamen (CPU), which have been shown to only receive V1 projections, thus further confirming that the labeled neurons were infected by the Hs06 virus in the V1 brain region. In addition, the authors conducted tracing experiments starting from the epithalamus (SC) and found that Hs06-labeled neurons project to many areas in the SC, including the pretectal nucleus and LGN, but not the V1 area. These indicate that Hs06 can undergo strictly anterograde transmonosynaptic tracing with the assistance of Her2CT9 and gD without retrograde infection or transsynapsis.
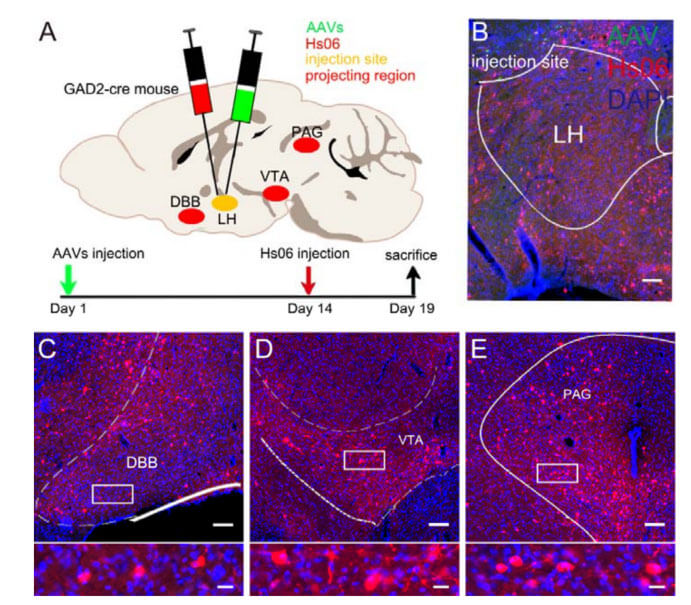
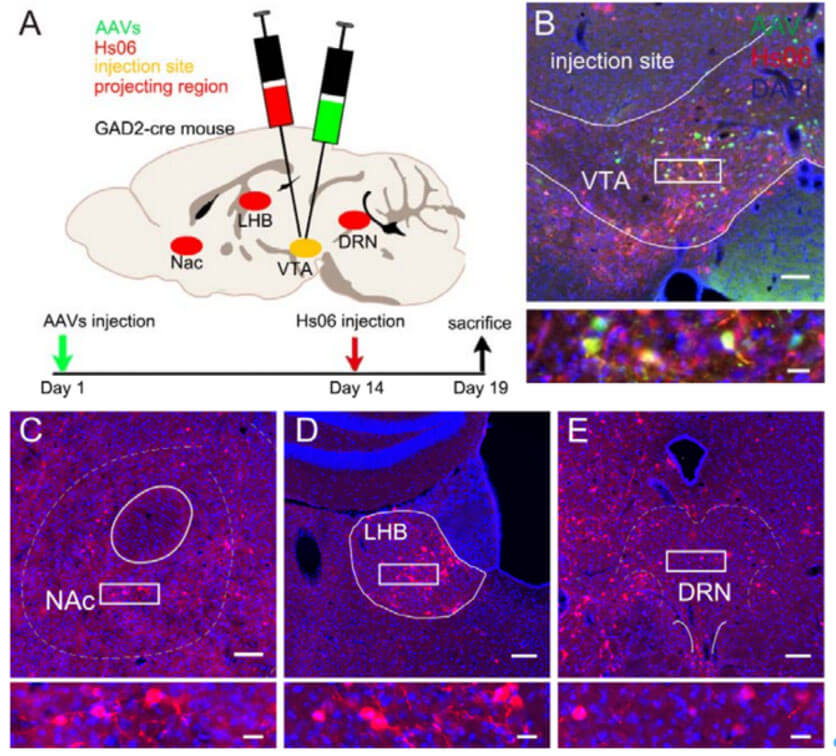
Accurate delineation of output neuronal circuits requires not only output pathways in a given brain region but also projection information from neurons in specific cell types. In animals, LH GABAergic neurons can project to multiple areas of the brain, including VTA, DBB, and PAG, thereby participating in a variety of instinctive behaviors of animals, such as eating, sleeping, and hunting. The authors used Hs06 virus to trace the direct output network of GABAergic neurons in the lateral hypothalamus (LH) or ventral tegmental area (VTA). The labeling results showed that the above brain areas (VTA, DBB, PAG) all receive LH GABAergic neurons. GABAergic neurons of the VTA can also project to many brain areas, such as the nucleus accumbens (NAc), ventral pallidum (VP), lateral hook ball (LHB), and dorsomedial raphe nucleus (DRN), and in the pressure and play an important role in animal behavior such as reward. The anterograde monosynaptic tracing ability of the Hs06 system was once again verified, and it also showed that the system has a relatively stable tracing ability.
In summary, the authors developed a strictly anterograde transmonosynaptic tracer based on the HSV1 H129 strain. Hs06 is a potential new strictly anterograde transmonosynaptic tracer that can effectively track the direct output pathways of specific neurons, expanding the current neural circuit tracer toolbox.