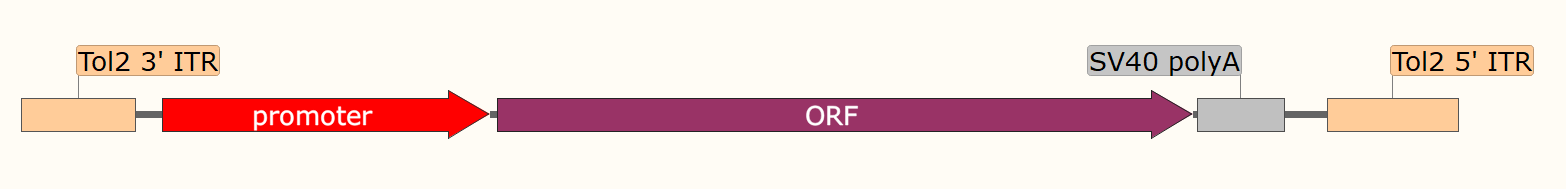
(2) Transgenic Animal Model Construction: The Tol2 transposon system can be used to construct transgenic animal models, such as zebrafish, medaka fish, Xenopus tropicalis, and mice, for studying the mechanisms of human disease development and drug screening.
Example 2: In 2010, researchers from the National Institute of Genetics in Japan published an article titled A simple and highly efficient transgenesis method in mice with the Tol2 transposon system and cytoplasmic microinjection [3], which introduced a new transgenic mouse preparation method based on the Tol2 transposon system — Tol2-mediated cytoplasmic injection (Tol2:CI). The article discusses in detail its role in improving transgenic efficiency and constructing mouse models. The Tol2 transposon system, through transposase, recognizes the ITRs and efficiently inserts the exogenous gene into the genome. The study designed three experimental conditions:Pronuclear injection (PNI), Tol2 pronuclear + cytoplasmic injection (Tol2:PNI+CI), Tol2 cytoplasmic injection (Tol2:CI), The results showed that the Tol2:CI method achieved a total transgenic efficiency of over 20%, which is much higher than the traditional pronuclear injection method (about 2%–5%). Additionally, the cytoplasmic injection method caused less embryo damage, had a higher survival rate, and showed higher integration frequency with high DNA and mRNA concentrations. The Tol2 system is not only applicable to model organisms such as zebrafish but also works well for mammals such as mice. Transgenic mice generated by the Tol2:CI method express the exogenous gene correctly, and these genes are passed on to the next generation through the germline. The Tol2 system demonstrated efficiency, stability, and ease of use in constructing transgenic mouse models, providing new technical means for genetic research and functional genomics.
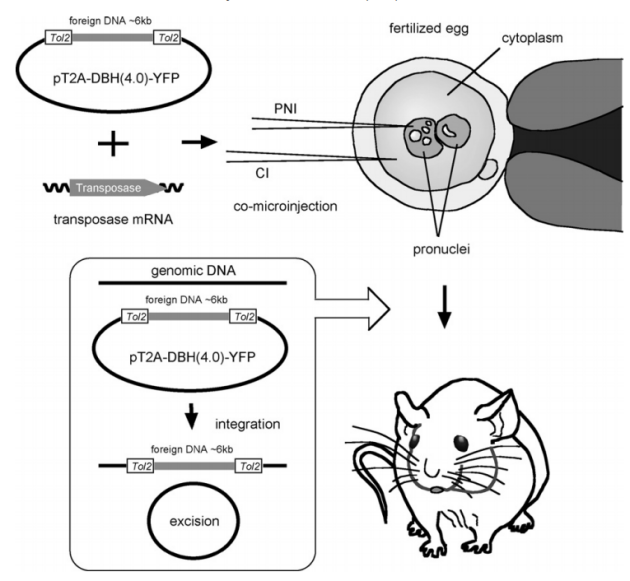
Figure 4: Schematic Diagram of Transgenic Mouse Model Construction Based on the Tol2 Transposon System (PNI: Pronuclear Injection; CI: Cytoplasmic Injection)
(3).Gene Therapy: The Tol2 transposon system can permanently integrate therapeutic genes into the target cell genome, providing a new tool for gene therapy of genetic diseases, tumors, and other conditions.
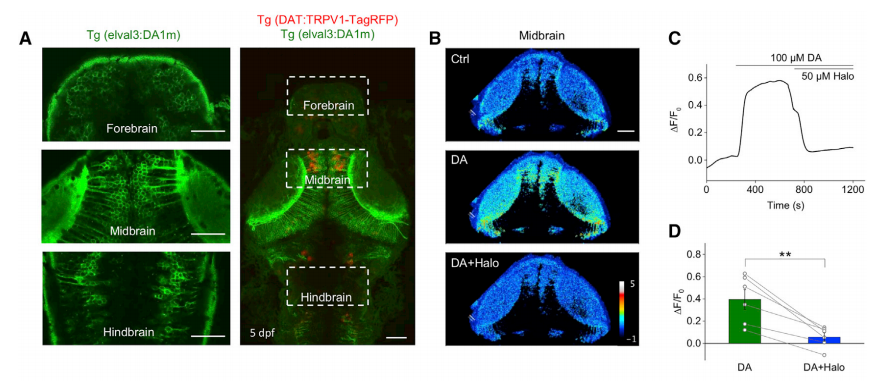
Example: Proteinuria is an important marker of kidney dysfunction and is typically assessed by measuring the protein content in urine, serving as a key clinical indicator of kidney disease. In 2022, a research team led by Rachel Lennon at the University of Manchester published an article online titled A novel nanoluciferase transgenic reporter to measure proteinuria in zebrafish [4], in which they used the Tol2 system to create a transgenic zebrafish line that stably expressed NL-D3. This system allows the quantification of proteinuria levels by measuring the luminescence intensity in the embryo culture medium using a fluorometer. In the experiment, the researchers successfully induced proteinuria by knocking down genes associated with kidney disease and treating zebrafish embryos with chemicals. They detected an increase in proteinuria using the NL-D3 reporter system. Additionally, the researchers used CRISPR-Cas9 technology to knock down the Col4a3 and Col4a4 genes, simulating the pathological features of Alport syndrome, and validated the potential of the NL-D3 system in drug screening. In conclusion, the Tol2 transposon system played a critical role in this study, enabling the stable integration of the NL-D3 reporter gene into the zebrafish genome and its specific expression in the liver, providing a reliable tool for detecting proteinuria, a key marker of kidney dysfunction.
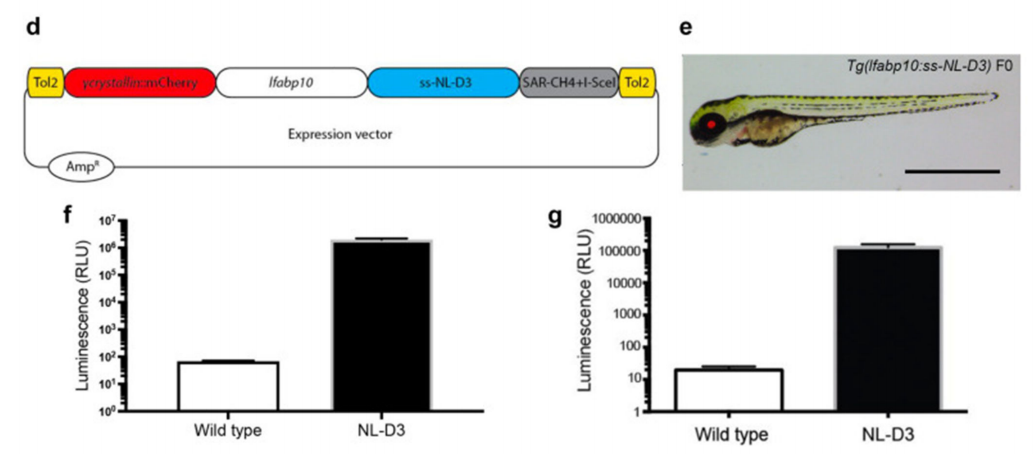
Figure 5: Construction of the NL-D3 Transgenic Zebrafish Line Based on the Tol2 Transposon System
References
[1]Wells, J. N. & Feschotte, C. A Field Guide to Eukaryotic Transposable Elements.Annu. Rev. Genet.54, 539–561 (2020).
[2]Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, Yong Z, Gao Y, Peng W, Wang L, Zhang S, Du J, Lin D, Xu M, Kreitzer AC, Cui G, Li Y. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell. 2018 Jul 12;174(2):481-496.e19.
[3]Sumiyama K, Kawakami K, Yagita K. A simple and highly efficient transgenesis method in mice with the Tol2 transposon system and cytoplasmic microinjection. Genomics. 2010 May;95(5):306-11.
[4]Naylor RW, Lemarie E, Jackson-Crawford A, Davenport JB, Mironov A, Lowe M, Lennon R. A novel nanoluciferase transgenic reporter measures proteinuria in zebrafish. Kidney Int. 2022 Oct;102(4):815-827.
Continuous Updates – Stay Tuned!
After delving into the Tol2 transposon, are you curious about other "jumpers" in the genome? The transposon family is vast, with each member having unique transposition mechanisms and application advantages. In addition to the Tol2 transposon, other transposons like PiggyBac and Sleeping Beauty also play a crucial role in genetic engineering. In the upcoming articles, Brain Case will introduce these transposons in detail, exploring their principles, applications, and advantages. Stay tuned for more insights into the fascinating world of transposons!
The Tol2 transposon system, known for its efficiency and flexibility, has become an important tool in gene editing and gene therapy. Brain Case now offers Tol2 transposon system-related gene overexpression services, providing a one-stop solution from vector construction, cell transfection, to stable cell line selection. Researchers are welcome to consult and discuss Via Email: bd@ebraincase.com