- E-mail:BD@ebraincase.com
- Tel:+8618971215294
In the previous article, we demonstrated the ability of AAV11 to infect microglia in different brain nuclei. In this article, we provide a detailed introduction to this tool in conjunction with specific products.
This product is an adeno-associated virus (AAV) vector based on the AAV11 serotype, capable of efficiently and specifically transducing microglia. It provides a powerful genetic manipulation tool for in-depth studies of microglial function, mechanisms, and their roles in neurological diseases.
Table 1: Backbone and Application
| Backbone | Usage |
| ITR | Terminal inverted repeat sequences forming a T-shaped hairpin structure |
| mIBA1 | Microglia-specific promoter |
| EGFP | Green fluorescent protein |
| WPRE | Enhancer to strengthen gene transcription |
| miR-9.T | MiRNA-9 targeting sequence to inhibit the expression of neuron-related genes |
| poly(A) | Transcription termination element of the gene |
Microglia are closely related to human health and the pathogenesis of various neurological disorders. Current research on microglia often relies on transgenic mice to introduce exogenous genes or genetic modifications into microglia, a method that is time-consuming, labor-intensive, and inefficient. Recombinant adeno-associated virus (rAAV) vectors have been developed as valuable tools to overcome these limitations, allowing for sufficient payload expression in microglia. Compared to previous studies, this product has the following features:
1. Specific Labeling: The rAAV11 product enhances specific transduction of microglia by carrying a specific mIBA1 promoter and targeting sequence, reducing nonspecific infection of other cell types such as neurons.
2. High Transduction Efficiency: The rAAV11 product shows high transduction rates in microglia when injected in situ in different brain regions and the spinal cord.
3. Editability with Broad Applications: By adding essential elements for optogenetics and chemogenetics, it can be applied to the fine analysis of neural circuits.
4. Customizability to Meet Personalized Needs: Structural modifications can be made to meet various needs, offering multiple options such as overexpression of target genes.
The rAAV11 product has successfully labeled microglia in various brain regions through targeted brain injections, including the Caudate Putamen (CPu), Dentate Gyrus of the Hippocampus (DG), Substantia Nigra Reticulata (SNr), and Primary Somatosensory Cortex (SSp).
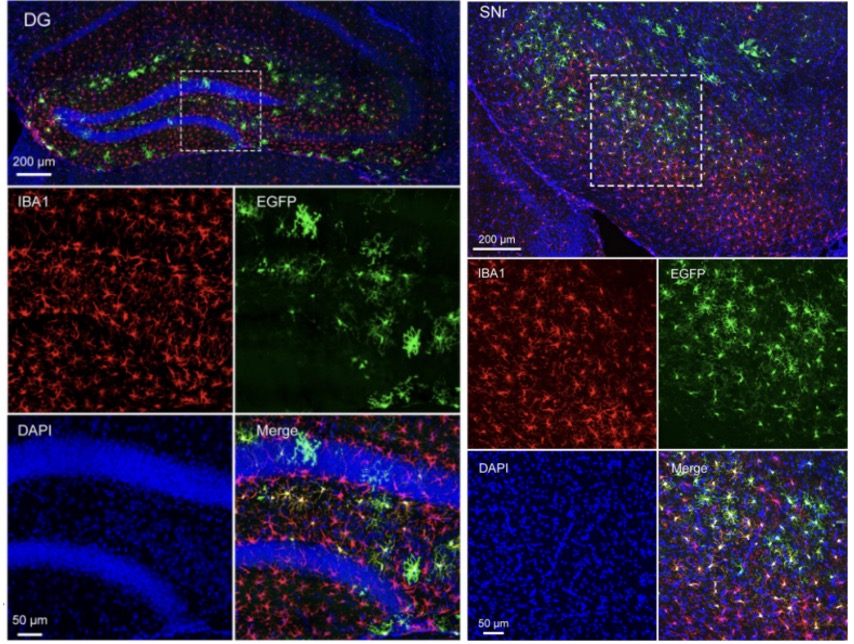
Figure 1: Labeling of Microglia in the Brain DG and SNr Regions
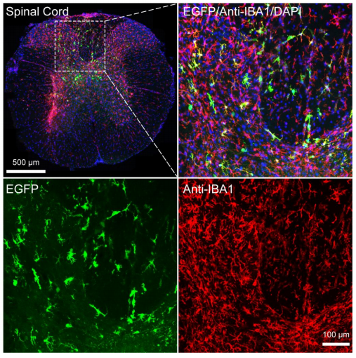
Figure 2: Labeling of Microglia in the Spinal Cord
Sparse labeling is achieved by reducing the viral injection volume, allowing for more detailed observation and analysis of microglial morphology.
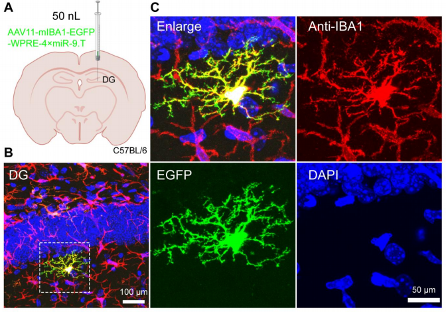
Figure 3: Sparse Labeling of Microglia in the Brain DG Region.
Table 2. Recommended Titer, Dosage, and Sample Collection Time for Product 6BC-2A0182:
| Brain Region/Nucleus | Title(VG/mL) | Injection Volume(nL) | Viral Expression Time |
| Caudate Putamen (CPu) | 1.00E+13 | 200 | 3weeks |
| Dentate Gyrus of the Hippocampus (DG) | 1.00E+13 | 200 | 3weeks |
| Substantia Nigra Reticulata (SNr) | 1.00E+13 | 200 | 3weeks |
| Primary Somatosensory Cortex (SSp) | 1.00E+13 | 200 | 3weeks |
| Spinal Cord | 2.50E+13 | 200 | 3weeks |
| Sparse Labeling in the DG | 1.00E+13 | 50 | 3weeks |
The rAAV11 product has made significant progress in efficiently and specifically transducing microglia. Building on this, techniques such as genetic regulation, optogenetics, and calcium imaging can be combined to further investigate microglial function, disease mechanisms, and explore their potential applications in neuroscience research and clinical therapy.
Q1: How long does the viral expression in the brain persist after injection?
A1: Generally, viral expression can persist for 3-4 weeks. Our experimental results are based on samples collected from mice 3-4 weeks after viral injection.
Q2: Microglia are immune cells, and in scientific research, we are often interested in whether they are in an activated or resting state. Will the viral injection activate microglia?
A2: Currently, our focus is on the transduction efficiency of microglia, and we have not yet assessed whether the labeled microglia are in an activated state. We plan to investigate this through single-cell sequencing in the future. Please stay tuned for updates.
Q3: The viral production titer is 1E+13 VG/mL. Is further dilution recommended, and is the injection volume 200 nL for all brain regions?
A3: We recommend using the titer of 1E+13 VG/mL without further dilution. The recommended injection volume is 200 nL, but it can be adjusted based on the size of the brain region.
Q4: If 200 nL of virus is injected in situ into the CPu region of the mouse brain, will the labeling of microglia completely diffuse throughout the entire CPu region?
A4: 200 nL of virus may not be sufficient to label microglia throughout the entire CPu region. For complete labeling of microglia across the whole CPu area, it is recommended to increase the injection volume and perform multiple injections at different sites.
Q5: Does the virus only have EGFP as the fluorescent marker, or are other options such as mCherry available?
A5: Currently, the product is in the trial phase and only includes EGFP as the fluorescent marker. If there is a specific need, you can contact us for custom options. We will also introduce additional choices based on high customer demand.
Ref:
[1] Nengsong Luo, Kunzhang Lin, Yuxiang Cai, Xiaokai Sui, Zilian Zhang, Jiayong
Xing , Gangning Liu, Wenjia Yuan, Jie Wang, Fuqiang Xu. Microglia-specific transduction via AAV11 armed with IBA1 promoter and miRNA-9 targeting sequences. bioRxiv preprint doi: https://doi.org/10.1101/2024.07.09.602653.