- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Recombinant adeno-associated virus (rAAV) has been widely used as a tracing tool for neural circuit research and as a gene therapy delivery vector. The diverse serotypes endow it with various tissue-targeting infection properties, attributed to the differences in interactions between AAV capsid proteins and cell surface receptors. Therefore, selecting the appropriate serotype according to experimental needs is crucial to meet specific exogenous gene delivery requirements.
AAV13 is renowned for its limited spread within the CNS, making it an ideal vector for precise labeling and delivery in small brain nuclei regions. However, the level of target gene expression mediated by AAV13 needs further improvement. To address this issue, the team led by Fuqiang Xu and Kunzhang Lin at the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, upgraded and modified AAV13. In June 2024, they published a research paper titled "Engineered AAV13 Variants with Enhanced Transduction and Confined Spread" in Zoological Research, reporting the development of two superior AAV13 mutants, named AAV13-587-7m8 and AAV13-585-7m8. Compared to AAV13, AAV13-587-7m8 and AAV13-585-7m8 exhibit higher transduction efficiency in the central nervous system of C57BL/6 mice, with AAV13-587-7m8 still retaining a limited spread range. These engineered AAV13 variants can accurately deliver exogenous genes to small or larger brain nuclei regions, making them excellent tools for precise in situ labeling in brain parenchyma. They hold significant application potential in gene therapy and neuroscience research.

Firstly, AAV13-CMV-EGFP and its mutants were injected into the primary somatosensory cortex (SSp). As shown in Figure 1, they exhibited confined spread, with a small cluster of in situ infected neurons displaying green fluorescence in a 1/6 brain section image. Compared to AAV13, AAV13-587-7m8 and AAV13-585-7m8 enhanced the expression intensity of the green fluorescent protein gene. AAV13-587-7m8 maintained a spread range similar to AAV13, while AAV13-585-7m8 had a larger spread range, making the two mutants suitable for gene delivery to brain regions of different sizes.
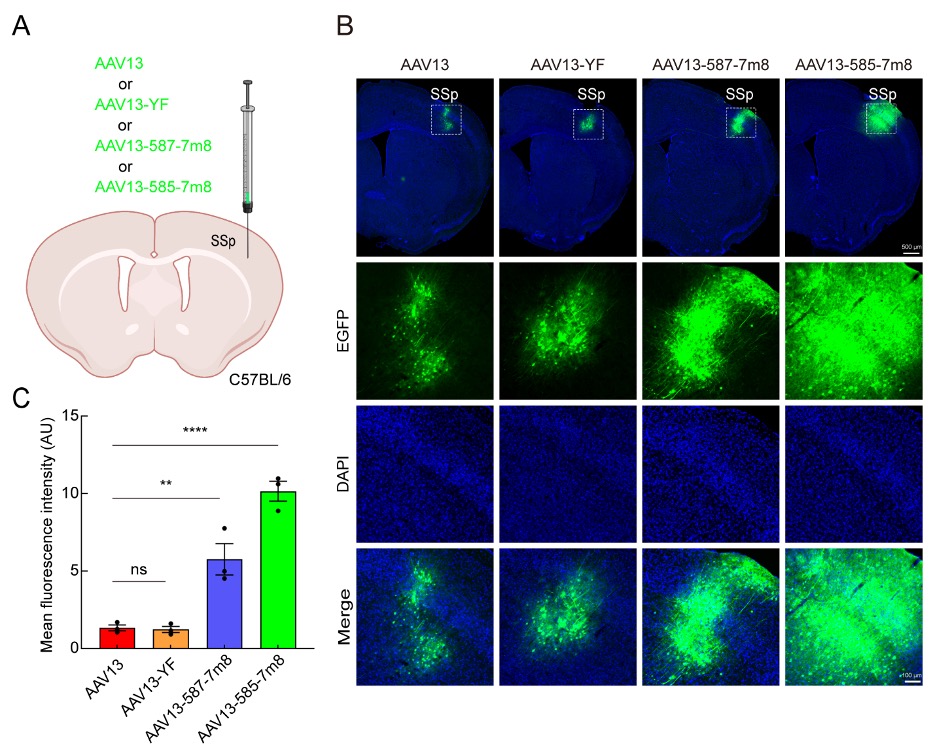
Figure 1: Efficient In Situ Transduction of the Primary Somatosensory Cortex by AAV13 Mutants
Secondly, to evaluate the gene delivery efficiency of AAV13 mutants in subcortical brain regions, equal amounts of AAV13 and its mutants were injected into the ventral tegmental area (VTA). As shown in Figure 2, the results were similar to cortical injections. AAV13-587-7m8 and AAV13-585-7m8 enhanced the expression intensity of the green fluorescent protein gene compared to AAV13. AAV13-587-7m8 maintained a spread range similar to AAV13, while AAV13-585-7m8 had a larger spread range, further demonstrating that these two mutants can be used for gene delivery to brain regions of different sizes.
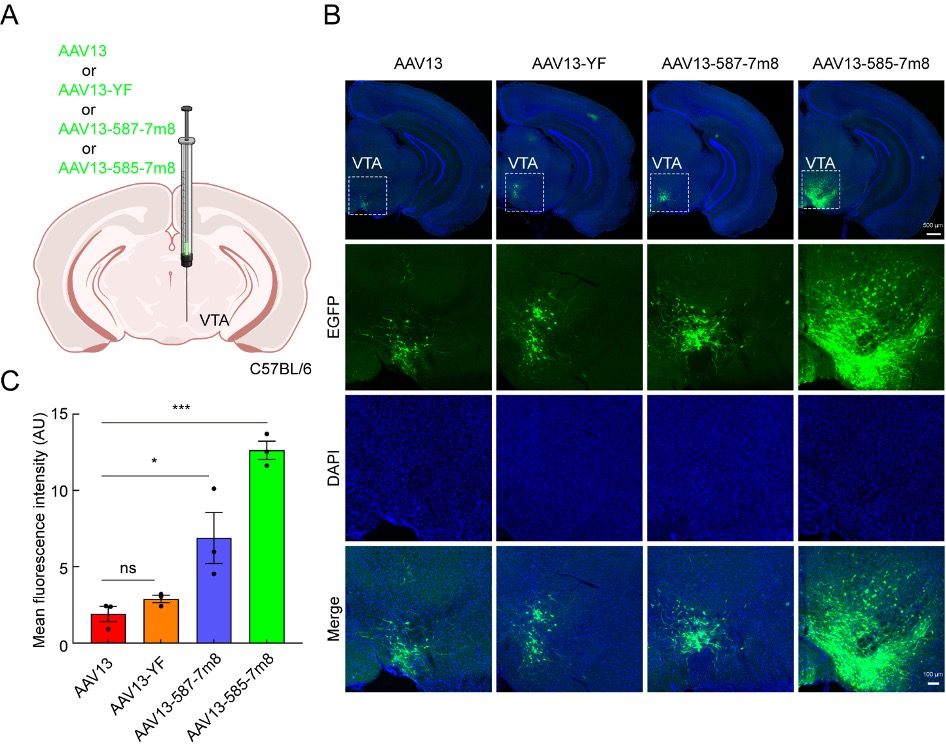
Figure 2: Efficient Transduction of the Ventral Tegmental Area by AAV13 Mutants
The injection results in the SSp and VTA brain regions showed that AAV13-587-7m8 could be used for efficient gene transduction in small brain regions. To evaluate the transduction performance of AAV13-587-7m8 at low doses, we reduced the injection dose of AAV13 and AAV13-587-7m8 from 200 nL to 50 nL, injecting them into the primary motor cortex (MOp). As shown in Figures 3A-C, after reducing the injection dose, the number of cells transduced by AAV13 was limited, and the mediated fluorescence expression intensity was very weak. In contrast, AAV13-587-7m8 still mediated strong fluorescence expression while maintaining a limited spread range. To further evaluate whether the transduction effect of AAV13-585-7m8 at a reduced dose could rival that of AAV13-587-7m8 at the unchanged dose, we diluted the viral titer of AAV13-585-7m8 by half and kept the titer of AAV13-587-7m8 unchanged, injecting 200 nL of each into the primary somatosensory cortex (SSp). As shown in Figures 3D-G, after reducing the dose, AAV13-585-7m8 still had a larger spread range but mediated weaker average fluorescence intensity compared to AAV13-587-7m8.
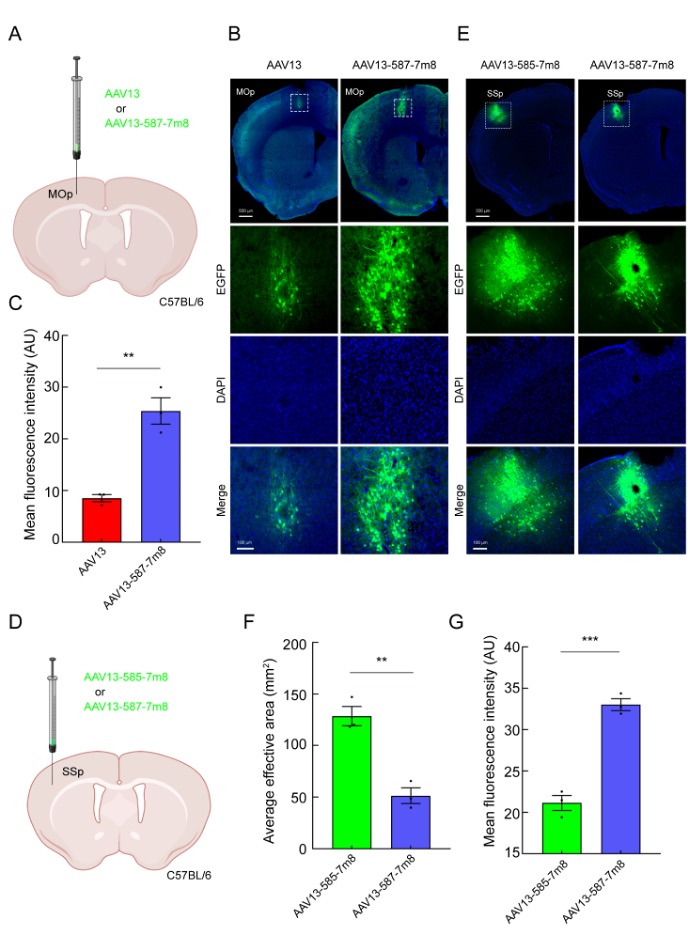
Figure 3: AAV13-587-7m8 Achieves High-Efficiency Transduction at Low Doses
Using AAV13-587-7m8 carrying Cre recombinase and comparing it with AAV13-Cre, the dual-virus labeling strategy (AAV13-587-7m8-Cre and AAV9-CAG-DIO-EGFP) enables sparse labeling of neurons at precise sites. AAV-Cre acts as a "controller" to lock the expression of the reporter protein in a small cluster of neurons. By controlling the dilution titer, the sparsity of infected neurons can be achieved, enabling "precise sparsity." In combination with the high-intensity fluorescence expression of "amplifiers" like AAV9-DIO-EGFP, "bright labeling" can be achieved (Figures 4A, 4B). At the same dilution factor, AAV13-587-7m8 labeled more neurons, indicating that further dilution of AAV13-587-7m8 can achieve a sparse labeling effect similar to AAV13.
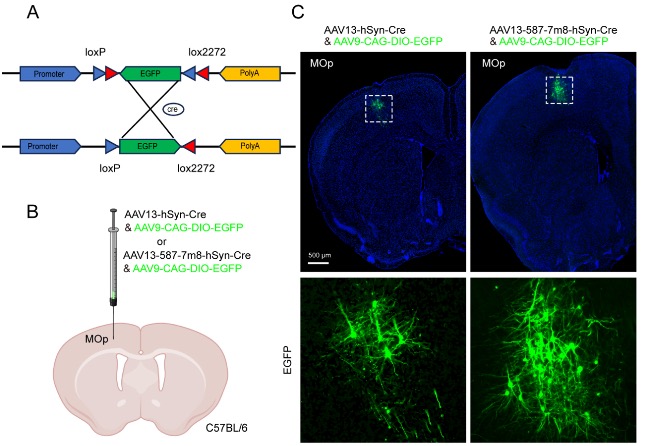
Figure 4: Sparse Labeling Based on a Dual-Virus Strategy
These studies confirmed that the two AAV13 mutants, AAV13-587-7m8 and AAV13-585-7m8, obtained through capsid protein modification, can serve as novel in situ gene delivery vectors. Compared to AAV13, both mutants enhanced the expression intensity of exogenous genes and are suitable for gene delivery to small and larger brain nuclei regions, respectively. Additionally, AAV13-587-7m8 can be used for high-brightness sparse labeling of neurons, facilitating the analysis of neuronal fine structures. Further exploration is needed to uncover the specific mechanisms underlying the different performances of these two mutants obtained through different site engineering modifications.
Please refer to the original article for more details.
Click to fill in the requirements and submit them to us!