- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Chemical genetics refers to the modification of biological macromolecules to enable them to interact with small molecules that were previously unrecognizable, thereby achieving controlled and reversible manipulation of the activity of biological macromolecules (associated with the addition or removal of compounds to initiate or interrupt specific reactions), and thereby specifically influencing physiological activities. The technology of Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), which are receptors activated solely by specific drugs, is currently the most widely used chemical genetics technology. In 2007, it was invented and utilized by members of Professor Roth's laboratory. Among them, hM3Dq and hM4Di are two of the most widely used chemical genetic manipulation tools in the field of neuroscience.
However, the DREADD system has its limitations: drugs cannot directly act on ion channel proteins but instead signal through G protein-coupled receptors as "messengers," resulting in unstable effects and potential side effects. Therefore, the use of biotechnology to directly modify ion channel proteins (Engineered Ligand-Gated Ion Channels, LGICs) as chemical genetic manipulation tools, such as PSAM4-5HT3 and PSAM4-GlyR, has gradually become a new chemical genetic tool used by researchers. These tools offer advantages such as ligand stability, rapid pharmacokinetics, and direct drug action on ion channel proteins.
In 2011, Scott Sternson's team at the Howard Hughes Medical Institute introduced the PSAM/PSEM chemical-genetic tools in the journal Science [1]. The PSAM component of this system is a modified ligand-binding domain (LBD) of the α7 nicotinic acetylcholine receptor (α7nAChR), while the PSEM agonist is the effector molecule that binds to PSAM. The principle of this system involves fusing the LBD of α7nAChR with diverse ion-channel proteins (IPDs) to confer selectivity for chloride ions, calcium ions, or other non-specific cations. In the study, the LBD of α7nAChR was fused with the IPDs of the 5-hydroxytryptamine 3a receptor (5-HT3) or the glycine receptor (GlyR), resulting in hybrid channels (α7-5HT3; α7-GlyR) with cationic or chloride ion conductance properties, respectively. These channels can be directly activated by the exogenous drug nicotine, and PSEM can directly control the opening and closing of ion channels, further regulating neuronal activity.

Fig1: DOI: 10.1126/science.aav5282
Subsequently, on April 12, 2019, the team led by Scott Sternson published a research paper titled "Ultrapotent Chemogenetics for Research and Potential Clinical Applications" in the journal Science [2]. Building upon their earlier PSAM/PSEM system design, this study employed bioengineering techniques to develop a set of neuro-targeted, non-invasive drug screening methods. They identified the low-toxicity PSEM agonist Varenicline (Varenicline, an FDA-approved non-nicotine smoking cessation drug) as readily penetrable to brain tissue. By altering amino acid compositions, they developed PSAM components with the highest selectivity and efficacy for Varenicline: PSAM4-5HT3 for neuron activation and PSAM4-GlyR for neuron inhibition.
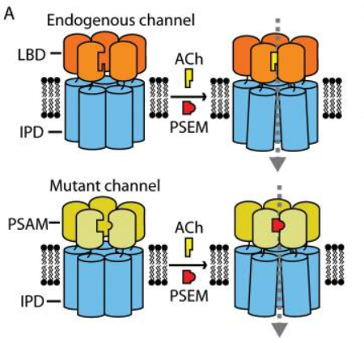
Fig 2: Schematic representation of the PSAM/PSEM system
PSAM4-5HT3 is an engineered receptor that fuses the ligand-binding domain (LBD) of the modified α7 nicotinic acetylcholine receptor with the ion pore domain (IPD) of the 5-hydroxytryptamine 3a receptor. It exhibits cation-selective conductance properties characteristic of the 5HT3 receptor. Upon activation by the agonist Varenicline, it depolarizes the cell membrane, leading to action potential generation.
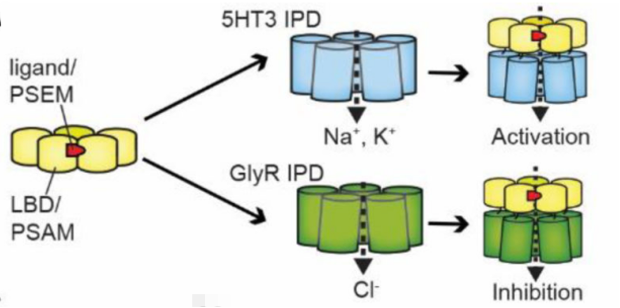
Figure 3: Schematic diagram of modular PSAM4-IPD chimeric channels engineered for cellular activation and inhibition.
PSAM4-GlyR, on the other hand, is an engineered receptor that fuses with the IPD of the glycine receptor. It possesses chloride-selective conductance properties. Upon activation by the agonist Varenicline, it hyperpolarizes the cell membrane, inhibiting neuronal action potential firing.
In the study, the authors validated the overexpression of PSAM4-5HT3 and PSAM4-GlyR receptor proteins in mouse neurons. The results indicated that under continuous exposure to low-dose Varenicline agonist (15nM), PSAM4-5HT3 could depolarize the cell membrane of dissociated hippocampal neurons in vitro, resulting in action potential firing, which could persist for at least 14-22 days. On the other hand, PSAM4-GlyR strongly inhibited firing due to shunting currents, even at a depolarizing current of 500pA, preventing neuronal firing for 3-18 days.
For further in vivo validation, the authors targeted GABAergic neurons in the substantia nigra pars reticulata (SNr) of mice and neurons in the globus pallidus interna (GPi) of macaque monkeys to overexpress PSAM4-GlyR. The study demonstrated that PSAM4-GlyR exerted significant inhibitory effects on neurons in both mice and macaque monkeys, with Varenicline doses required being 5-10 times lower compared to other drugs. Low-dose agonists had minimal impact on rodent and primate models, exhibited rapid efficacy, and were effective across their brains.
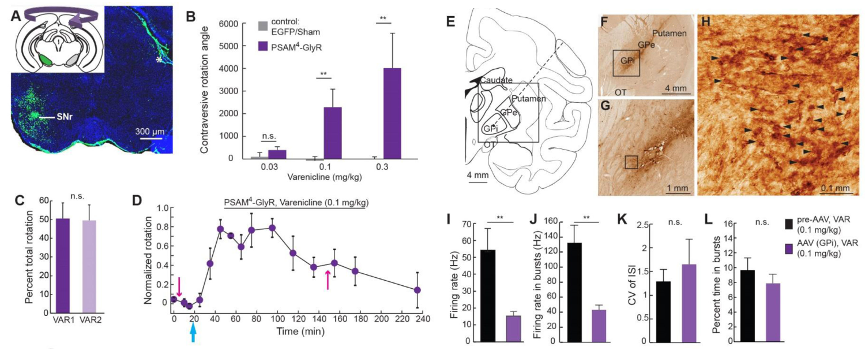
Fig 4: Overexpression of PSAM4-GlyR in mouse and rhesus monkey neurons.
Summary: PSAM4-5HT3 and PSAM4-GlyR are novel chemical-genetic tools based on LGICs technology. Their chemical-inducible drugs can directly act on ion channel proteins, providing advantages such as stability, rapid efficacy, and minimal side effects. In conclusion, PSAM4-5HT3 and PSAM4-GlyR offer new tools for studying brain function and hold promise for the future development of safe and effective treatments for neurological disorders.
literature citation
1.Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011 Sep 2;333(6047):1292-6. doi: 10.1126/science.1206606.
2.Magnus CJ, Lee PH, Bonaventura J, Zemla R, Gomez JL, Ramirez MH, Hu X, Galvan A, Basu J, Michaelides M, Sternson SM. Ultrapotent chemogenetics for research and potential clinical applications. Science. 2019 Apr 12;364(6436):eaav5282. doi: 10.1126/science.aav5282.
Product information
| Product Type | Product Number | Name(Backbone) |
| Chemical Genetics - Chemical Activation | BC-2637 | rAAV-CAG-EGFP-T-PSAM4-5HT3 HC |
| BC-3097 | rAAV-hSyn-DIO-PSAM4-5HT3 HC-P2A-mCherry | |
| BC-3098 | rAAV-EF1α-DIO-PSAM4-5HT3 HC-IRES-EGFP | |
| BC-3100 | rAAV-hSyn-PSAM4-5HT3 HC-P2A-mCherry | |
| Chemical Genetics - Chemical Inhibition | BC-2638 | rAAV-CAG-mCherry-T-PSAM4-GlyR |
| BC-3031 | rAAV-hSyn-DIO-PSAM4-GlyR-P2A-EGFP | |
| BC-3099 | rAAV-EF1α-DIO-PSAM4-GlyR-IRES-mCherry | |
| BC-3101 | rAAV-hSyn-PSAM4-GlyR-P2A-EGFP | |
| BC-2694 | rAAV-CaMKIIα-PSAM4-GlyR-IRES-mCherry |