- E-mail:BD@ebraincase.com
- Tel:+8618971215294

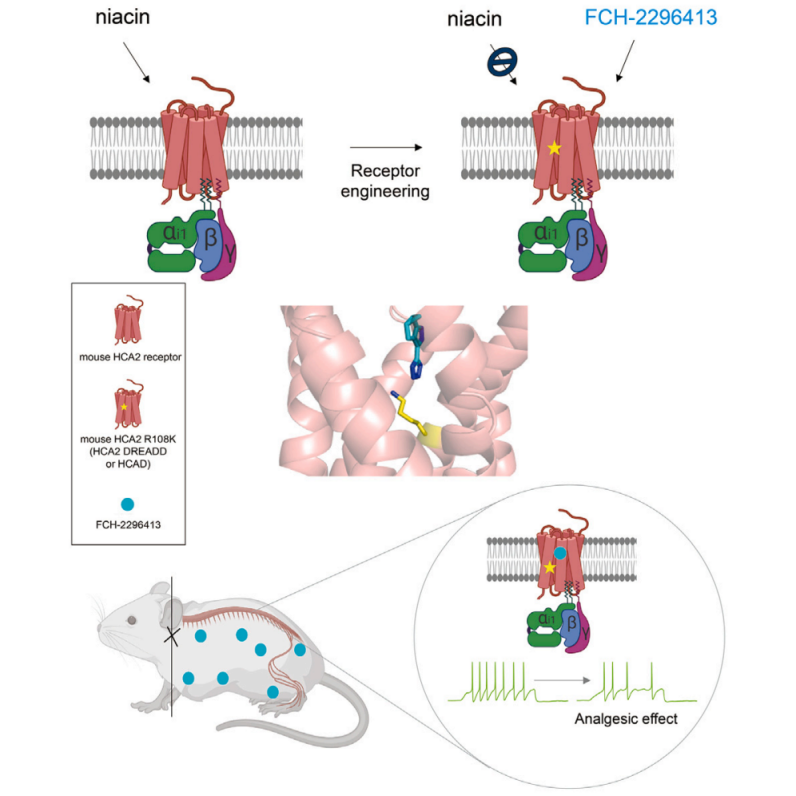
Most acute, perioperative, and chronic pain is driven by peripheral primary afferent neurons in the DRG. Therefore, reducing DRG neuron activity, particularly nociceptor activity, is a key strategy for developing advanced pain treatments.
To verify the in vivo functionality of the HCAD DREADD system, researchers developed an adeno-associated virus (AAV) to infect DRG nociceptors and express mHCAD-mCitrine in a Cre recombinase-dependent manner (PHP.S-CAG-FLEx-mHCAD-mCitrine). In newborn (P2) Avil-Cre and TRPV1-Cre mice, AAV was administered via intraperitoneal injection, while in adult mice, it was delivered intrathecally to label DRG neurons and DRG nociceptors, respectively. Following viral injection, mHCAD-mCitrine was successfully expressed in Cre+ DRG neurons (Figure 2A).
At the cellular level, whole-cell patch-clamp recording was performed on isolated DRG neurons to assess the function of mHCAD-mCitrine. In DRG neurons, Gi signaling typically inhibits voltage-gated calcium channels (VGCCs), thereby reducing neurotransmitter release and suppressing the transmission of somatosensory information—including pain signals—from the peripheral nervous system (PNS) to the spinal cord and brain.
In Avil-Cre mice, FCH-2296413 reduced voltage-activated calcium current amplitudes in HCAD-mCitrine+ neurons in a dose-dependent manner. At a concentration of 100 nM, FCH-2296413 showed no inhibitory effect, whereas at 1 μM and 10 μM, it significantly inhibited VGCC activity (Figures 2B and 2C). Furthermore, FCH-2296413 slowed calcium current kinetics after administration (Figures 2B and 2D).
These inhibitory effects closely resemble those produced by opioid analgesics acting on endogenous Gi-GPCRs in DRG neurons. Notably, in mHCAD-mCitrine-negative (control) neurons, FCH-2296413 had no effect on VGCC activity.
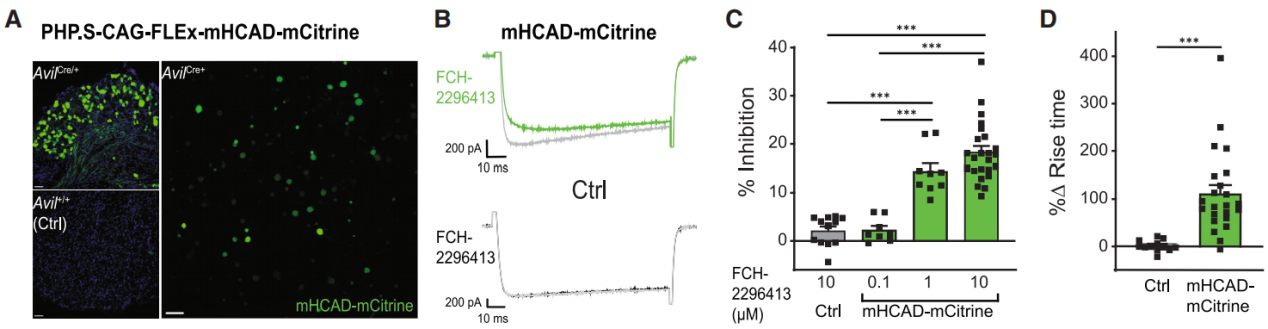
Figure 2 Whole-Cell Patch-Clamp Recording of Voltage-Activated Calcium Currents
Another typical effect of Gi signaling is membrane hyperpolarization, which leads to reduced excitability and altered somatosensory encoding. The study found that FCH-2296413 significantly decreased the resting membrane potential (RMP) of mHCAD-mCitrine+ DRG neurons (Figures 3E and 3F) and reduced action potentials (APs) (Figures 3E and 3G).
These effects were reversible, as RMP and APs returned to baseline levels after washing out FCH-2296413 (Figures 3F and 3G). Additionally, no such changes were observed in mHCAD-mCitrine-negative control neurons (Figures 3F and 3G).
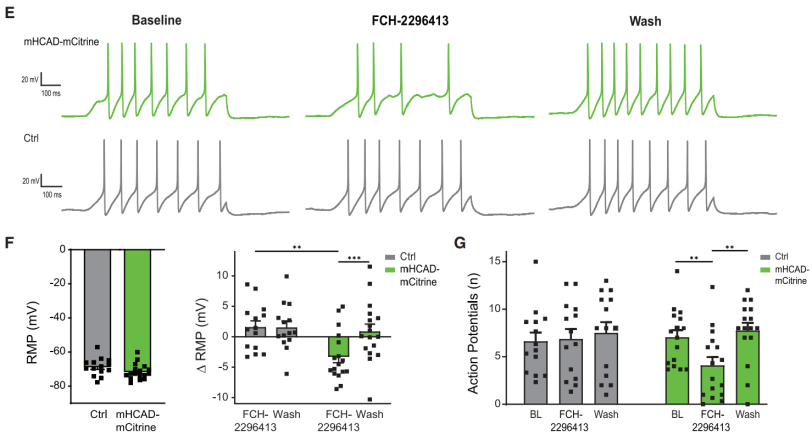
Figure 3 Effects of the mHCAD System on Resting Membrane Potential and Action Potentials
Previous reports have suggested that hM4Di expression can alter the function of ion channels in DRG neurons even in the absence of an agonist. Therefore, to further characterize mHCAD function and its potential advantages in studying peripheral neurons, researchers examined whether mHCAD expression affected ion currents involved in AP formation in the absence of FCH-2296413.
The results showed no significant differences in AP waveform threshold, overshoot (membrane potential exceeding the normal resting potential at the AP peak), or duration (Figures 4H-4M, 4I-4K). This indicates that voltage-gated sodium channels and voltage-gated potassium channels responsible for AP initiation, rise, and fall phases were not affected by mHCAD expression.
However, mHCAD expression slightly increased the amplitude of afterhyperpolarization (AHP) without altering its decay time (Figures 4L and 4M). Most importantly, there was no difference in excitability between mHCAD-mCitrine+ cells and non-expressing cells (Figure 4N). Additionally, unlike hM4Di, mHCAD expression did not induce any detectable gene expression changes (Figure 4O).
In summary, mHCAD expression had minimal impact on DRG neuron function in the absence of FCH-2296413, whereas FCH-2296413 activation of mHCAD influenced classical Gi signaling pathways associated with reduced excitability and neurotransmitter release in DRG neurons.
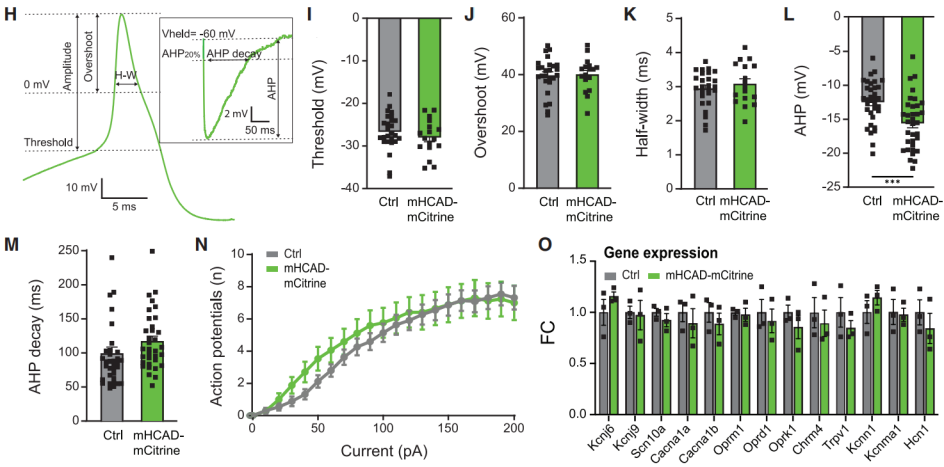
Figure 4 Effects of mHCAD on Currents Involved in Action Potential Formation
Researchers evaluated the effects of FCH-2296413 (10 mg/kg, subcutaneous injection) on pain perception in Trpv1-Cre mice that received an intrathecal injection of either PHP.S-CAG-FLEx-mHCAD-mCitrine or the control virus PHP.S-CAG-FLEx-tdTomato (Ctrl) (Figures 5A and 5B).
In the hot plate test for acute thermal pain (Figure 5C), FCH-2296413 significantly reduced the duration and frequency of forepaw licking, hindpaw licking, and jumping in mice expressing mHCAD in DRG nociceptors. It also increased the latency to the first nocifensive behavior, suggesting an analgesic effect mediated by mHCAD.
Furthermore, in the Freund’s adjuvant (CFA) model of tissue injury and chronic inflammatory pain (Figure 5D), FCH-2296413 alleviated mechanical and thermal hypersensitivity in mHCAD-expressing mice, but not in control mice, confirming the therapeutic anti-hyperalgesic properties of FCH-2296413 and mHCAD.
Overall, these findings demonstrate that in mice expressing mHCAD in DRG nociceptors, FCH-2296413 exerts analgesic effects on both physiological and pathological pain conditions.
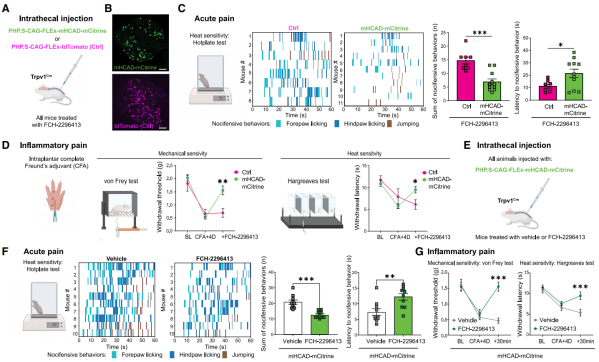
Figure 5 Peripheral Chemogenetic Analgesic Effects of mHCAD
Previous studies have reported that other chemogenetic drugs (such as CNO) can alter locomotor behavior in mice, potentially confounding interpretations of various behaviors, including nocifensive responses. To address this, researchers examined the effects of FCH-2296413 on various behaviors in wild-type (WT) mice that do not express mHCAD (Figures 6H-6P).
The results showed that FCH-2296413 had no effect on mechanical thresholds in WT mice and did not induce changes in nocifensive behaviors in the tail immersion test (Figure 6I), hot plate test (Figures 6J and 6K), or Hargreaves test.
Furthermore, researchers evaluated the effects of FCH-2296413 on locomotion and anxiety-like behavior in the open field test and motor coordination in the rotarod test. In these experiments, FCH-2296413 did not affect the behavior of WT mice.
These findings indicate that the peripheral DREADD system proposed in this study can specifically modulate DRG nociceptor function and alleviate pain. This system holds potential for mechanistic studies of peripheral neuron function and the treatment of diseases affecting the PNS.
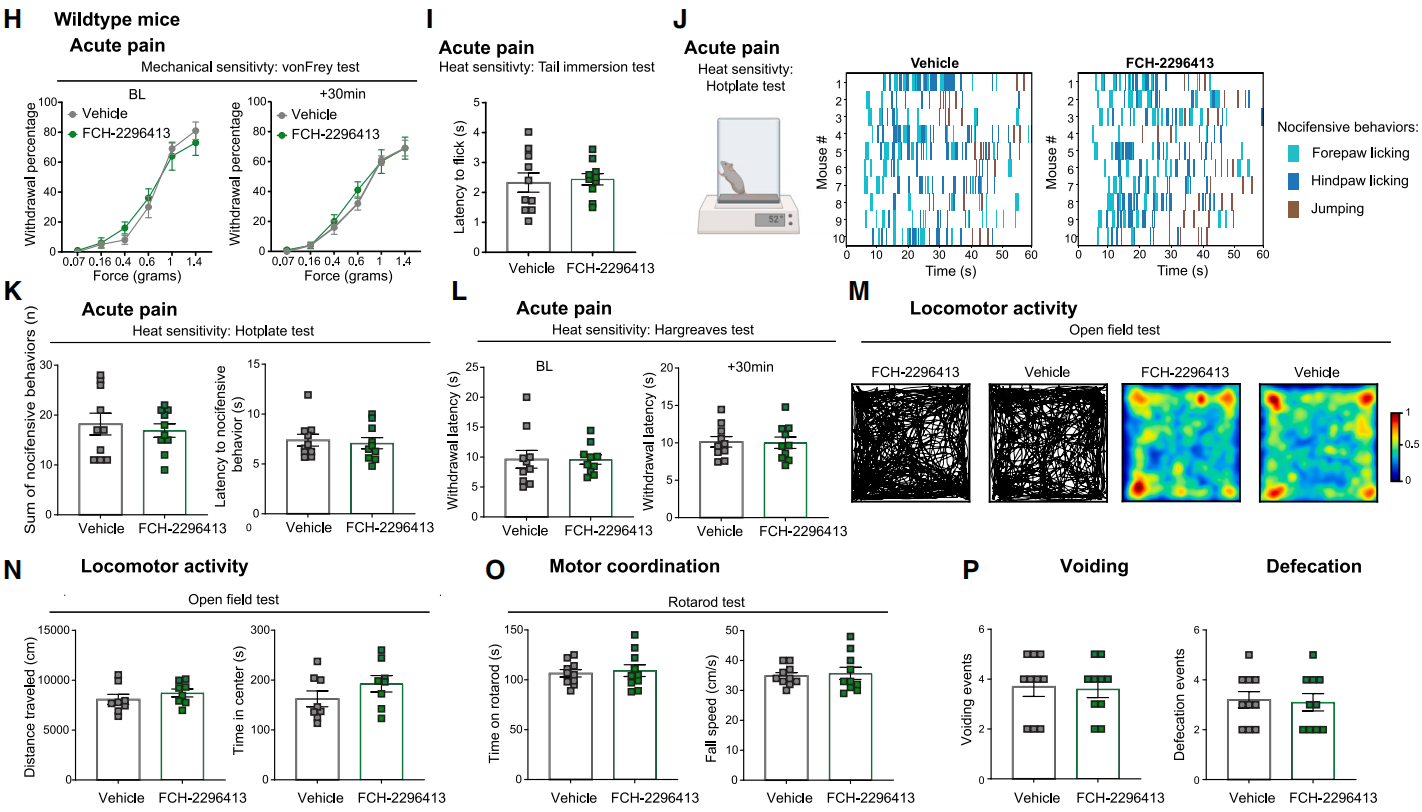
Figure 6 FCH-2296413 Does Not Affect Behavior in WT Mice
Brain Case can provide customers with a full range of vector construction, virus packaging and stable cell line construction services. If you are interested in customized services, please contact bd@ebraincase.com for details or to place an order.