- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Nowadays, metabolic diseases like obesity and diabetes have become global health challenges. The glucagon-like peptide-1 receptor (GLP-1R) is a peptide hormone receptor widely expressed in the body, involved in energy regulation and glucose homeostasis, and is closely associated with conditions such as obesity and diabetes. GLP-1R is also the target of some clinical weight-loss medications, such as semaglutide and liraglutide. However, despite the important clinical role of GLP-1R, there are still very few tools available for studying the dynamic activation of GLP-1R under high spatiotemporal resolution.
On June 2, 2023, researchers from the University of Zurich published a paper titled "Optical tools for visualizing and controlling human GLP-1 receptor activation with high spatiotemporal resolution" in eLife. The study developed a genetically encoded sensor, GLPLight1, with high sensitivity (ΔF/F0 = 528%) and temporal resolution (τON = 4.7 seconds). This sensor can respond to GLP-1 signals in real-time and at high spatiotemporal resolution, enabling the monitoring of the activation dynamics of the human GLP-1 receptor (GLP-1R).
The researchers designed the sensor based on the human GLP-1 receptor (hmGLP1R) by inserting a circular arrangement of green fluorescent protein (cpGFP) between the K336 and T343 amino acids in the third intracellular loop (ICL3) of hmGLP1R. This led to the development of an initial GLP-1 fluorescence sensor, which showed weak fluorescence response to GLP-1. The researchers then optimized the sensor with several modifications, including: 1)Removal of the endogenous GLP1R N-terminal secretion sequence (amino acids 1-23); 2)Introduction of an endoplasmic reticulum export sequence at the C-terminus to enhance membrane expression; 3)Mutations (L260K, K336Y, T343N) near ICL2 and cpGFP to improve fluorescence response to GLP-1; 4)Mutation of eight phosphorylation sites at the C-terminus responsible for GLP-1R internalization to reduce sensor protein internalization.
After optimization, they obtained GLPLight1, a fluorescence sensor with excellent membrane expression, high sensitivity (ΔF/F0 = 528%), and good temporal resolution (τON = 4.7 seconds). They also identified a mutant (L141A, N300A, E387A), which did not respond to GLP-1 but expressed well on the membrane, as a control sensor—GLPLight-ctr.
To further explore the expression and fluorescence response characteristics of GLPLight1, the researchers used adeno-associated viruses (AAVs) to express it in primary cortical neurons and 293T cells. The results showed that GLPLight1 was well expressed on the cell membrane and exhibited a maximal fluorescence response of 456% to GLP-1 (10 µM) in primary cortical neurons. They also measured the spectral characteristics of GLPLight1 in HEK cells, finding that its maximum excitation wavelength was 500 nm, and the maximum emission wavelength was 512 nm.
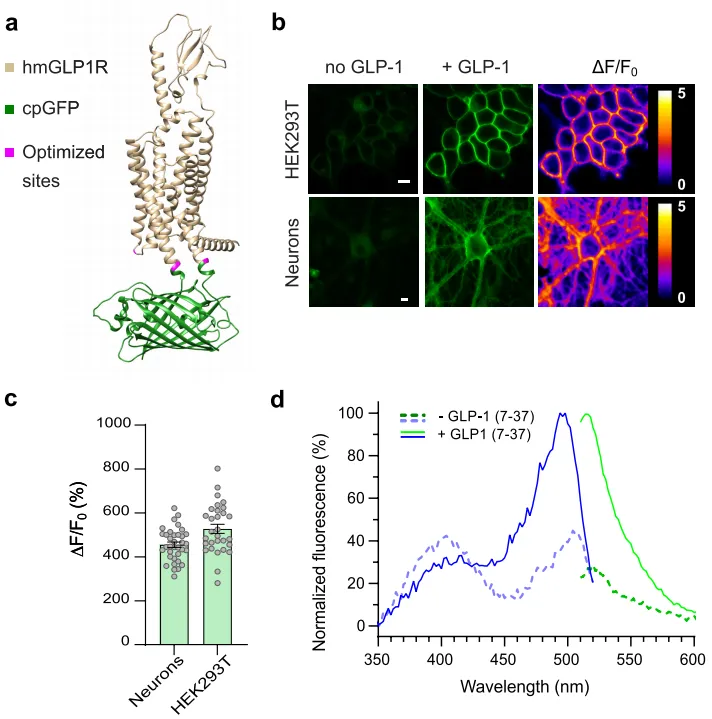
Figure 1. Structure and Optical Characteristics of GLPLight1
Additionally, using methods like luciferase complementation assays, the researchers compared the downstream signaling characteristics of GLPLight1 with endogenous GLP1R. The results showed that GLPLight1 significantly reduced coupling to multiple G proteins (such as miniGs, miniGq, miniGi). Under high concentrations of GLP-1 (100 nM), although GLPLight1 exhibited strong fluorescence response, there was no increase in downstream cAMP levels, suggesting that GLPLight1 does not participate in the activation of the cAMP signaling pathway. This indicates that GLPLight1 can serve as an effective tool for real-time monitoring of GLP-1R activation status without interference from other factors in the signaling process, and it does not affect normal signaling pathways.
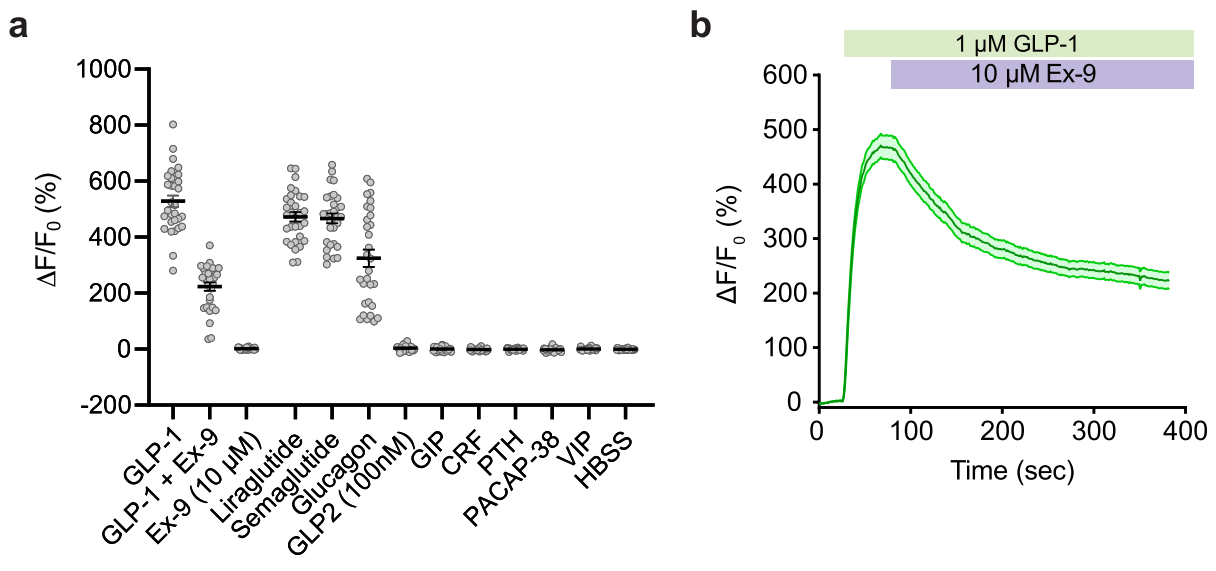
Figure 2. Pharmacological Properties of GLPLight1 in 293T Cells
To further verify whether GLPLight1 can sensitively detect the release of endogenous GLP-1 in vitro, the researchers co-cultured GLUTag cells (a cell line capable of secreting GLP-1/glucagon) with HEK293T cells expressing GLPLight1. After adding exogenous GLP-1, they observed that when HEK293T cells were co-cultured with GLUTag cells, the fluorescence response of GLPLight1 to GLP-1 was significantly reduced compared to the control group with only HEK293T cells. This indicates that GLPLight1 was pre-activated by the endogenous GLP-1 secreted by GLUTag cells. This result suggests that GLPLight1 can effectively detect the release of endogenous GLP-1 and could be used as a potential screening tool to study intrinsic and extrinsic factors that regulate the release of GLP-1 from enteroendocrine cells (ECs) in vitro.
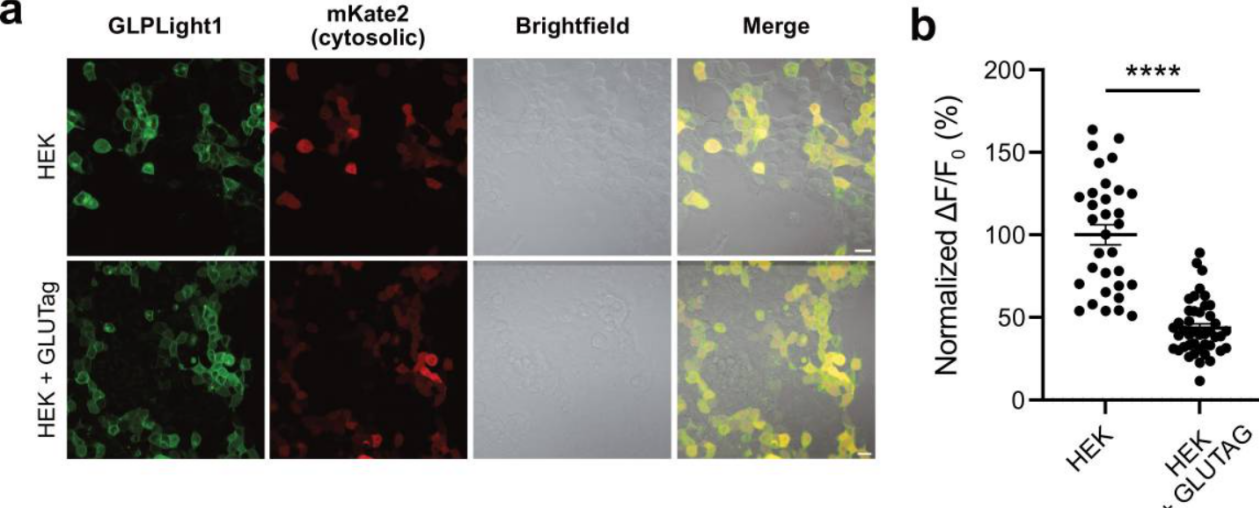
Figure 3. GLPLight1 Detects Endogenous GLP-1 Release in Cells
The researchers used GLPLight1 to establish an all-optical detection method to study the feasibility of optical control of GLP1R activation with photo-GLP1, a novel light-sensitive GLP-1 derivative. Photo-GLP1 is constructed by adding a photocaging molecule to the N-terminal of the GLP-1 peptide chain. Upon 20 minutes of LED light exposure (λ=370 nm, 0.64 mW/mm2), the wild-type GLP1 was generated. The results showed that after 10 seconds of ultraviolet light (405 nm) exposure, GLP1 was normally expressed, and the fluorescence of GLPLight1 increased significantly. Prolonged ultraviolet exposure (100 seconds) resulted in a higher degree of GLPLight1 response, indicating successful uncaging of GLP1 and activation of GLPLight1 sensors on the cells.
The researchers found that the response speed of GLPLight1 was affected by the presence of photo-GLP1. Photo-GLP1 significantly reduced the activation speed of the sensor, suggesting that photo-GLP1 may influence its activation kinetics by binding to the extracellular domain of GLP1R. Using GLPLight1, they also explored the spatial information of GLP1R activation after uncaging photo-GLP1, finding that GLPLight1 could detect GLP-1 responses with high spatial resolution, and this response could be confined to specific regions of the cell membrane. Finally, by using the cAMP fluorescence sensor G-Flamp1, the researchers confirmed that uncaged photo-GLP1 could control functional signaling after GLP1R activation, achieving optical control of GLP1R signaling with high spatiotemporal resolution.
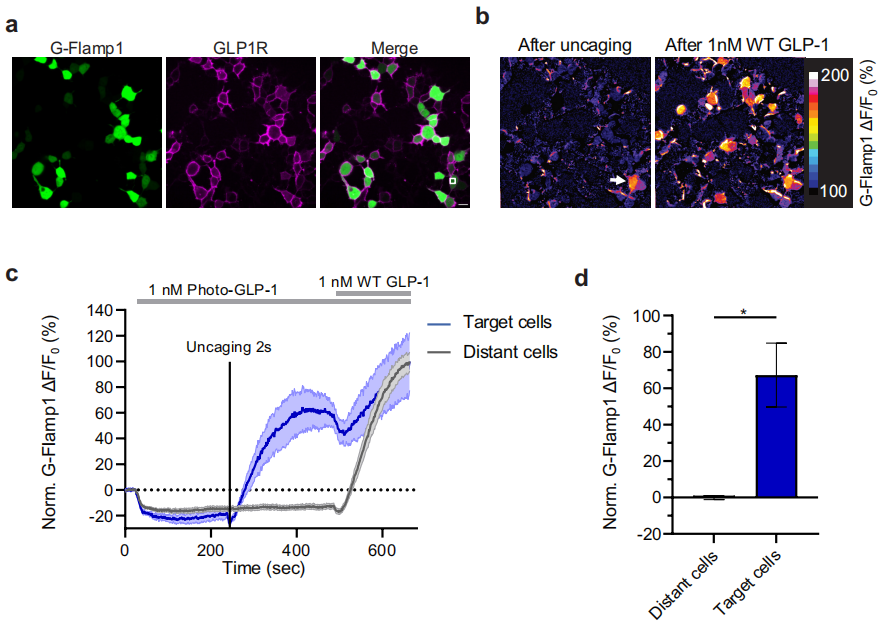
Figure 4. Impact of Uncaged Photo-GLP1 on Intracellular Signaling
This study developed a genetically encoded sensor, GLPLight1, with high sensitivity and temporal resolution, capable of real-time, high spatiotemporal resolution monitoring of GLP-1 signals and the activation dynamics of human GLP-1R. This provides an important tool for understanding the role of the GLP-1/glucagon/GLP1R signaling system in physiology, as well as promoting drug screening and development targeting the GLP1R pathway.