- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Recombinant adeno-associated virus (rAAV) vectors are among the preferred tools for human gene therapy applications due to their demonstrated safety in clinical trials. These vectors can transduce both dividing and non-dividing cells, induce minimal cytotoxicity, and enable long-term transgene expression. rAAV has been shown to efficiently infect various tissues, including the liver, lungs, eyes, and skeletal muscles. Tissue-specific transduction depends on capsid properties and the administration route.
Adult skeletal muscle accounts for 40-50% of body weight and comprises various distinct muscles. Each of these muscles consists of contractile muscle fibers, which are post-mitotic multinucleated cells surrounded by a specialized cell membrane—the sarcolemma—and a layer of extracellular matrix (ECM) known as the basal lamina. Studies focusing on systemic administration have primarily investigated the muscle tropism of rAAV serotypes: rAAV serotypes 1, 6, 7, 8, and 9 have shown effective transduction of skeletal muscle in most animal models.
The primary AAV serotypes targeting muscle include AAV1, AAV6, AAV8, and AAV9. Among them, AAV8 and AAV9 exhibit higher tissue specificity for skeletal muscle and cardiac muscle.
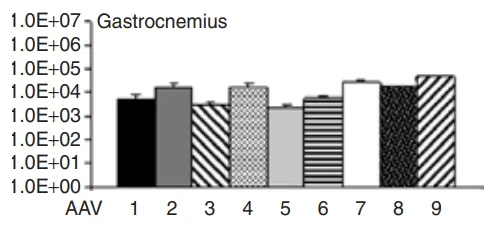
Figure 1. Expression Profiles of Different AAV Serotypes in the Gastrocnemius Muscle
When studying muscle tissue infection using viral vectors, two main administration methods are employed: systemic injection and intramuscular injection.
(1)Intramuscular Injection
This method typically involves injecting into the forelimb or hindlimb muscles of mice. The needle should be inserted vertically and quickly into the muscle, and the injection can proceed if no blood is observed. Intramuscular injection allows localized high-efficiency expression near the injection site. However, its main drawback is the limited spread of the virus, often requiring multiple injections, with each site typically receiving 5-10 µL.
(2)Tail Vein Injection
This approach involves injecting the virus into the tail vein of the animal to distribute it to muscles throughout the body. While this method achieves relatively uniform expression, a significant portion of the virus is absorbed by other organs, reducing the amount of virus that targets the muscles.

Serotypes: AAV1–AAV10
Promoter: CMV
Experimental Animals: 3-month-old male C57BL/6J mice
Injection Protocol: Tibialis anterior muscle (TA) injection at a dose of 3.5 × 10¹⁰ vg per mouse, expression assessed after 4 weeks.
Experimental Results:
AAV1–10 vectors (rAAV-CMV-LacZ, where LacZ encodes β-galactosidase) were locally injected into the tibialis anterior muscle of 3-month-old C57BL/6J male mice. Four weeks post-injection, β-gal staining revealed the highest expression of β-galactosidase in the TA muscle injected with the AAV9 serotype, compared to other serotypes. Additionally, AAV9-injected muscles showed the highest AAV genome copy numbers, indicating that AAV9 achieved the highest transduction efficiency in local skeletal muscle injection.
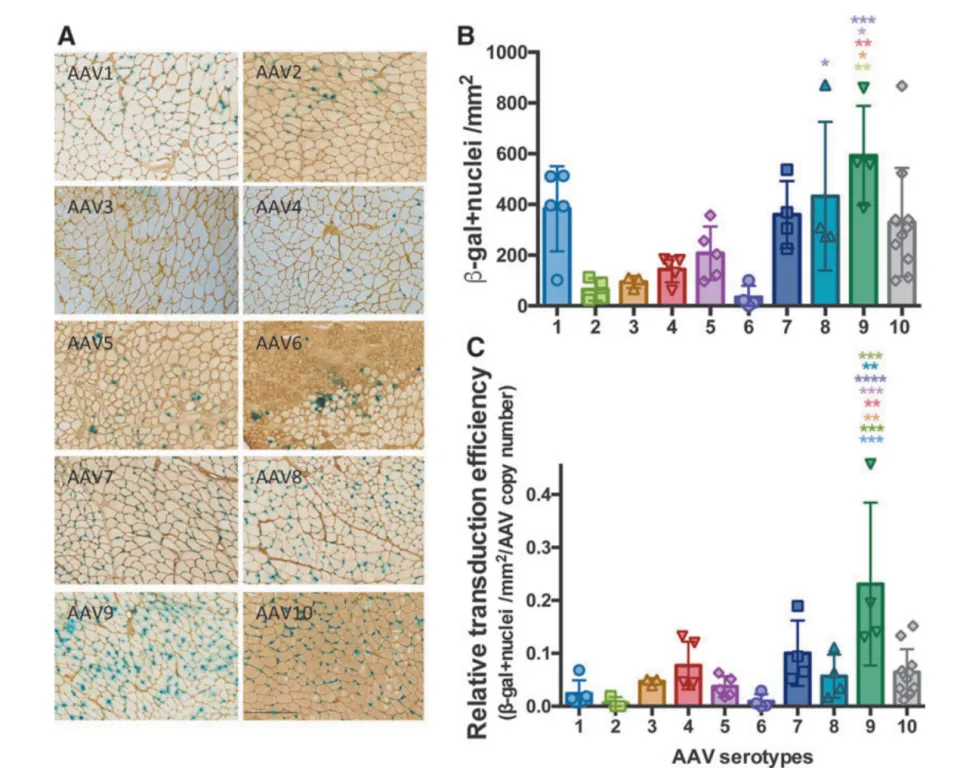
Figure 3. Comparison of AAV Transduction Efficiency in Muscle Tissue of C57BL/6 Mice
Serotypes: AAV9, MyoAAV1A
Promoter: CMV
Experimental Animals: C57BL/6J mice
Injection Protocol: Intravenous injection at a dose of 1.0 × 10¹² vg per mouse , expression assessed after 2 weeks.
Experimental Results:
To investigate the transduction profile and biodistribution of MyoAAV1A following systemic administration, adult C57BL/6J mice were injected with 1.0 × 10¹² vg (approximately 4.0 × 10¹³ vg/kg) of AAV9 or MyoAAV1A-CMV-EGFP. Two weeks post-injection, transgene expression and vector genome abundance were analyzed across various tissues. Tissue fluorescence imaging revealed stronger muscle fluorescence signals in mice injected with MyoAAV1A compared to AAV9. Notably, MyoAAV1A demonstrated superior transduction efficiency in the heart compared to AAV9. This finding is particularly significant, as the heart is both a critical and heavily affected organ in many hereditary muscular diseases.
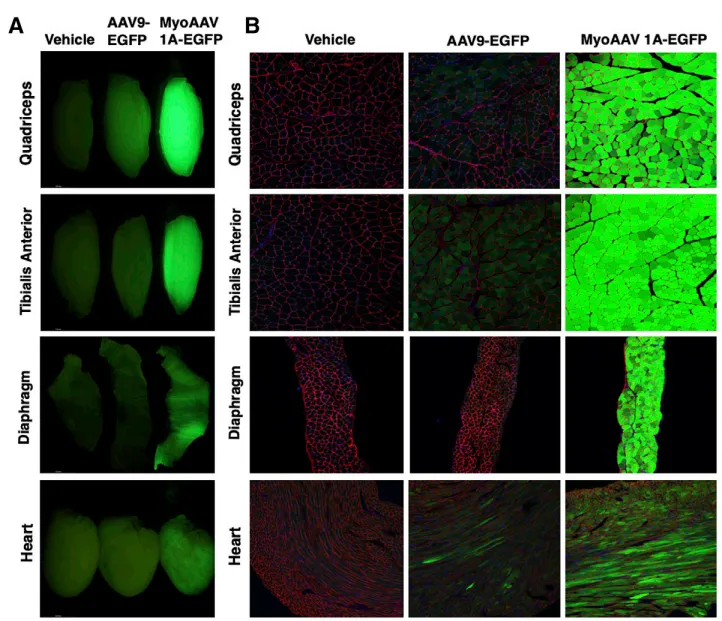
Figure 4. MyoAAV1A Efficiently Transduces Mouse Skeletal Muscle
Serotype: LICA1
Promoter: CMV, hACTA1
Experimental Animals: LGMDR3 mice (a muscular dystrophy model), 4 weeks old
Injection Protocol: Intravenous injection at a dose of 5.0 × 10¹² vg/kg, expression assessed after 4 weeks.
Experimental Results:
Low-dose AAV9 (encoding full-length human α-sarcoglycan (hSGCA) )or LICA1 virus (AAV-hACTA1-hSGCA), was injected into 4-week-old SGCA knockout mice (LGMDR3). After 4 weeks of treatment, analysis showed that in the three tested muscles (tibialis anterior (TA), quadriceps (Qua), diaphragm (Dia)), the LICA1 treatment group exhibited significantly higher AAV genome copy numbers, mRNA levels, and SGCA+ muscle fiber percentages compared to the AAV9 treatment group. In the more severely affected muscles, LICA1 demonstrated higher transduction efficiency than AAV9. Even with low-dose treatment, LICA1-treated mice showed more significant functional and histological recovery.
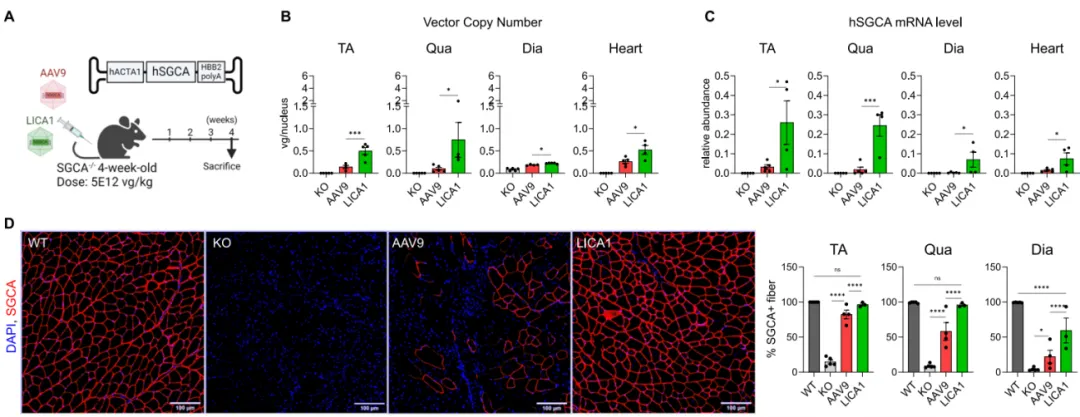
Figure 5. Low-Dose LICA1 Outperforms AAV9 in Restoring Muscular Dystrophy Phenotype and Function
Serotype: MyoAAV4A
Promoter: CMV, Spc5-12, Spc2-26
Experimental Animals: mdx mice (muscular dystrophy model), 3-4 weeks old
Injection Protocol: Retro-orbital intravenous injection at a dose of 8.0 × 10¹³ vg/kg, expression assessed after 5 weeks.
Experimental Results:
Duchenne muscular dystrophy (DMD) is a progressive muscular dystrophy caused by the complete absence of the dystrophin protein in muscle fiber membranes, leading to progressive muscle damage and weakness. To assess whether AAV-N1/M3/C6 (full-length FL-dystrophin gene fragment) treatment improved the muscle pathology in mdx mice, serum creatine kinase (CK) levels were measured 5 weeks after administration. Compared to wild-type (WT) mice, mdx mice had significantly elevated serum CK levels, which were notably reduced in the AAV treatment group, indicating that FL-dystrophin expression helped reduce muscle damage in dystrophic mice. Muscle contractility was also measured using an in vivo muscle testing system, showing a significant improvement in muscle tension in mdx mice treated with AAV-N1/M3/C6, compared to the control group.
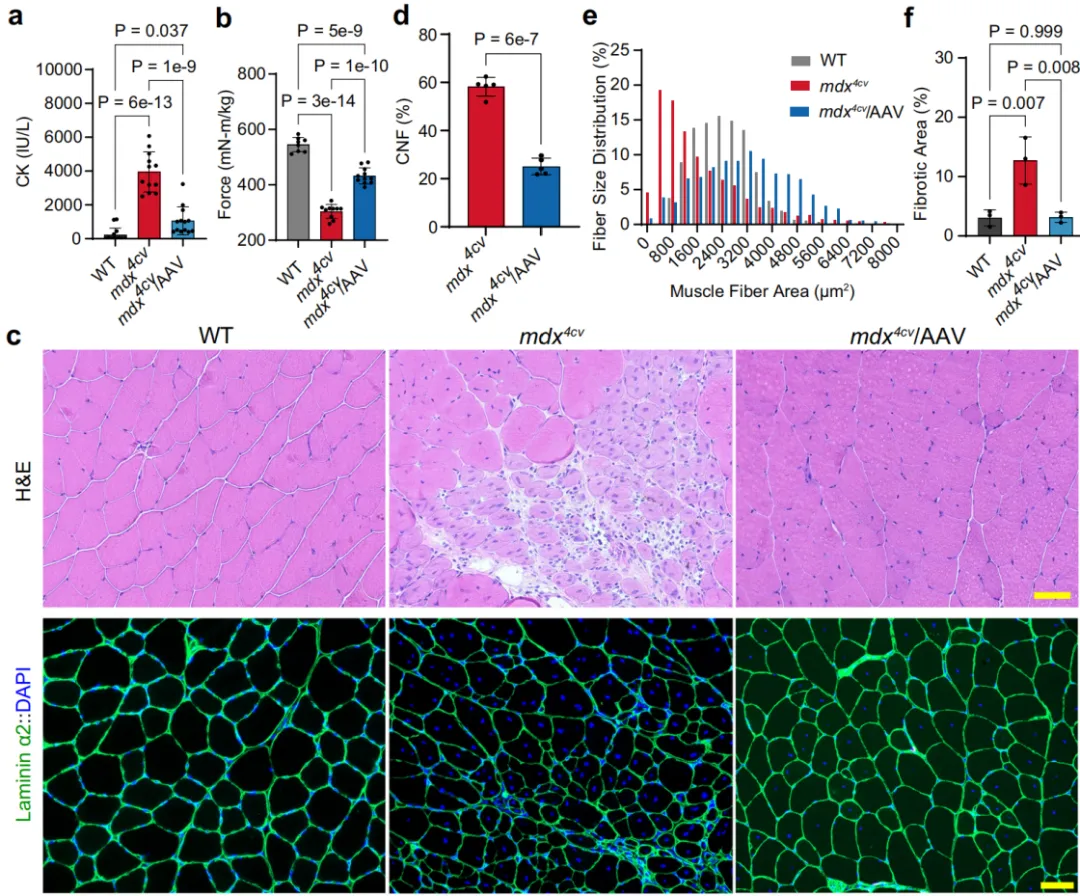
Figure 6. Muscle Function and Histopathological Changes in mdx Mice Following Systemic Injection of MyoAAV4A-Dys-N1/M3/C6
References
1、Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073-1080.
2、Tabebordbar M, Zhu K, Cheng JKW, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351(6271):407-411.
3、Muraine L, Bensalah M, Dhiab J, et al. Transduction Efficiency of Adeno-Associated Virus Serotypes After Local Injection in Mouse and Human Skeletal Muscle. Hum Gene Ther. 2020;31(3-4):233-240.
4、Tabebordbar M, Lagerborg KA, Stanton A, et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell. 2021;184(19):4919-4938.e22.
5、Vu Hong A, Suel L, Petat E, et al. An engineered AAV targeting integrin alpha V beta 6 presents improved myotropism across species. Nat Commun. 2024;15(1):7965.
6、Zhou Y, Zhang C, Xiao W, Herzog RW, Han R. Systemic delivery of full-length dystrophin in Duchenne muscular dystrophy mice. Nat Commun. 2024;15(1):6141.
Brain Case offers various virus packaging customization services. For more information, please contact bd@ebraincase.com.
This article is not authorized for reproduction. If needed, please contact us to obtain the original text.