1. AAV-PHP.B
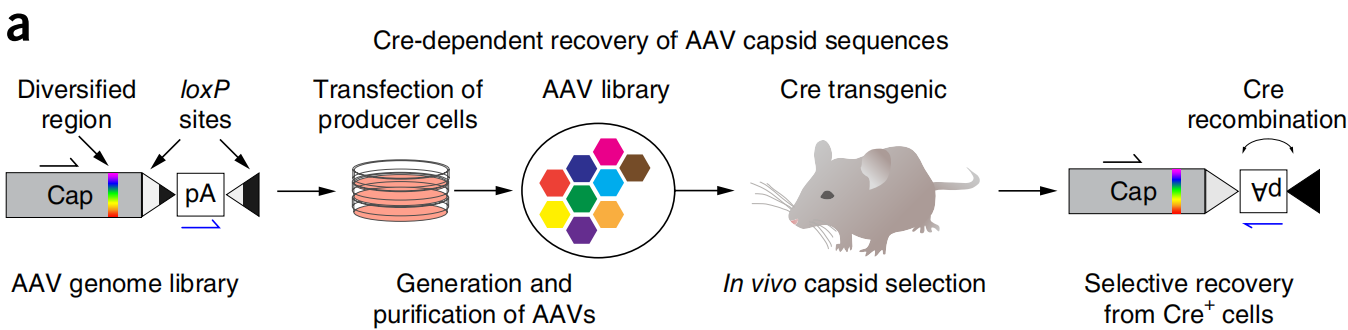
On February 1, 2016, the team led by Viviana Gradinaru at the California Institute of Technology published a research paper titled "Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain" in Nature Biotechnology. By applying the CREATE technology, the researchers successfully developed a novel adeno-associated virus (AAV) variant named AAV-PHP.B. When intravenously injected into adult mice, AAV-PHP.B can efficiently penetrate the blood-brain barrier and widely transduce the central nervous system (CNS). The researchers injected AAV-PHP.B carrying the green fluorescent protein (GFP) gene into the mice via the tail vein. This virus specifically entered and infected neurons in the brain. The experiments observed that AAV-PHP.B's transduction efficiency in the CNS was significantly higher than that of the control AAV-9 virus. It was able to effectively transduce various cell types in the CNS, including astrocytes, oligodendrocytes, and neurons. Compared to AAV-9, AAV-PHP.B's infection efficiency was increased by at least 40-fold, indicating a significant advantage in gene delivery to the CNS.
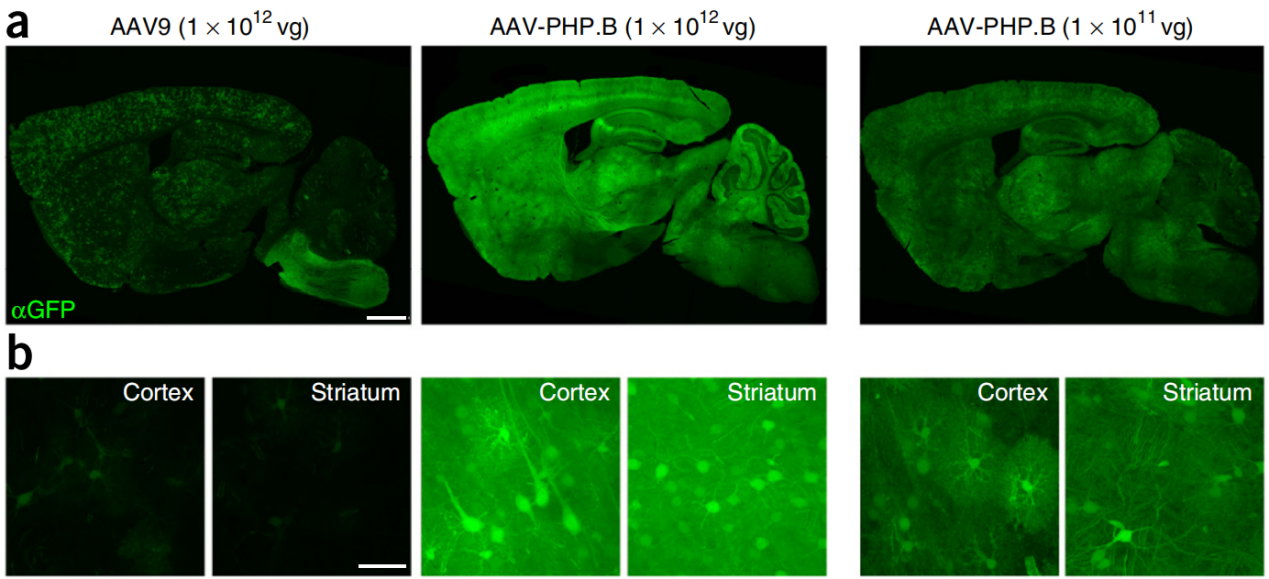 Figure 2: AAV-PHP.B mediated gene delivery to the CNS after intravenous injection in adult mice
Figure 2: AAV-PHP.B mediated gene delivery to the CNS after intravenous injection in adult mice
Three weeks after tail vein injection, the researchers detected strong expression of green fluorescent protein (GFP) in CNS regions such as the cerebral cortex, striatum, spinal cord, retina, thalamus, and cerebellum. Compared to AAV9, AAV-PHP.B showed reduced transduction efficiency in the pancreas and adrenal glands, while its expression in the liver, heart, muscle, and kidneys was comparable to that of AAV9, indicating similar transduction characteristics in these tissues.
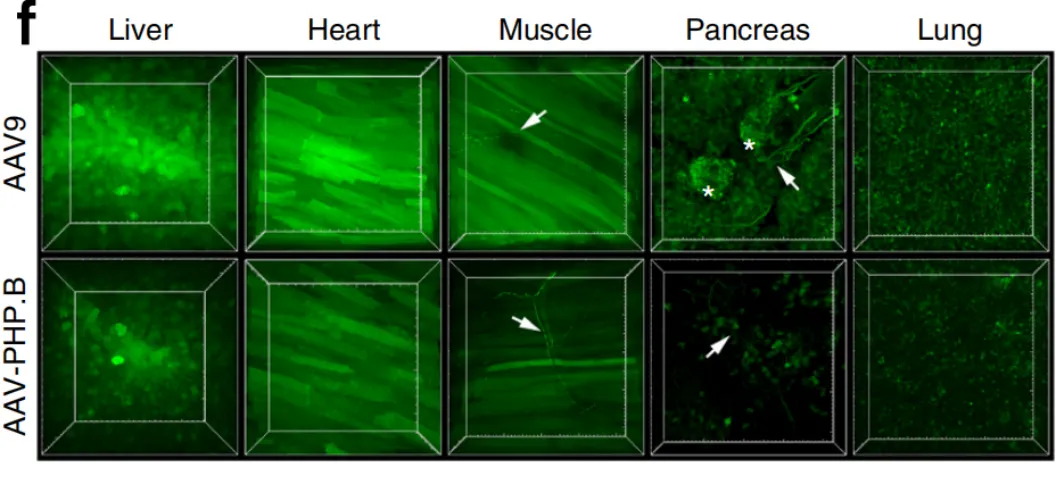 Figure 3: AAV-PHP.B mediated gene delivery to peripheral tissues after intravenous injection in adult mice
Figure 3: AAV-PHP.B mediated gene delivery to peripheral tissues after intravenous injection in adult mice
2.AAV-PHP.eB and AAV-PHP.S
On June 26, 2017, Viviana Gradinaru's team published a research paper titled "Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems" in Nature Neuroscience. The paper describes two novel adeno-associated virus (AAV) capsid variants, AAV-PHP.eB and AAV-PHP.S, designed for efficient transduction of the central nervous system (CNS) and peripheral nervous system (PNS), respectively. The researchers used the CREATE technology to evolve these new capsid variants through two rounds of in vivo screening from the parent capsids AAV-PHP.B and AAV9.
AAV-PHP.eB, when intravenously injected into adult mice at a total dose of 1 × 10^11 viral genomes (vg), was able to transduce 69% of cortical neurons and 55% of striatal neurons in the CNS. Compared to AAV-PHP.B, AAV-PHP.eB provided higher GFP fluorescence intensity and transduction efficiency in several brain regions.
AAV-PHP.S, when injected intravenously into adult mice at a total dose of 1 × 10^12 vg, was capable of transducing 82% of dorsal root ganglion (DRG) neurons as well as cardiac and intestinal neurons in the PNS. The transduction efficiency of AAV-PHP.S in the PNS was significantly higher than that of AAV9.
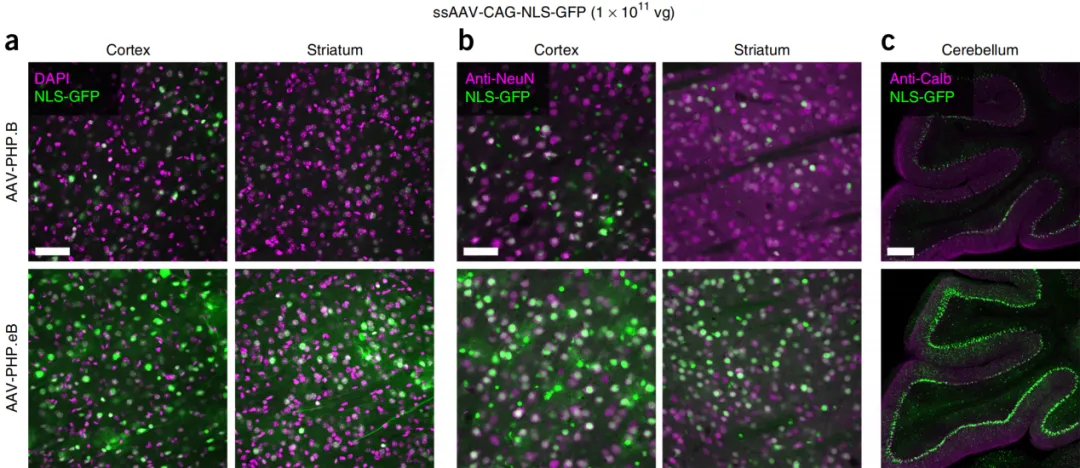
Figure 4:AAV-PHP.eB and AAV-PHP.B transduce the central nervous system.
3.AAV.CAP-B10 and AAV.CAP-B22
On December 9, 2021, Viviana Gradinaru's team published a research paper titled "AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset" in Nature Neuroscience. Using a Cre transgenic mouse screening platform and the M-CREATE (Multiplex Cre-dependent Evolution) method, the researchers performed sequence engineering on the AAV-PHP.eB capsid and developed new adeno-associated virus (AAV) capsid variants, AAV.CAP-B10 and AAV.CAP-B22. These variants achieved broad and specific gene delivery to the central nervous system (CNS) in mice and marmosets while reducing liver targeting.
AAV.CAP-B10 demonstrated similar transduction efficiency in the mouse brain and spinal cord compared to AAV-PHP.eB but significantly reduced liver transduction. AAV.CAP-B22 exhibited 12 times higher transduction efficiency in the brain compared to AAV9, with liver expression similar to AAV9. In terms of cell type specificity, AAV.CAP-B10 showed high specificity for neurons in the CNS, with lower transduction efficiency for astrocytes and oligodendrocytes. AAV.CAP-B22, on the other hand, displayed widespread cell type transduction in the marmoset brain. These AAV variants enabled extensive CNS transduction in marmosets with reduced liver expression, offering new possibilities for gene therapy in non-human primate models.
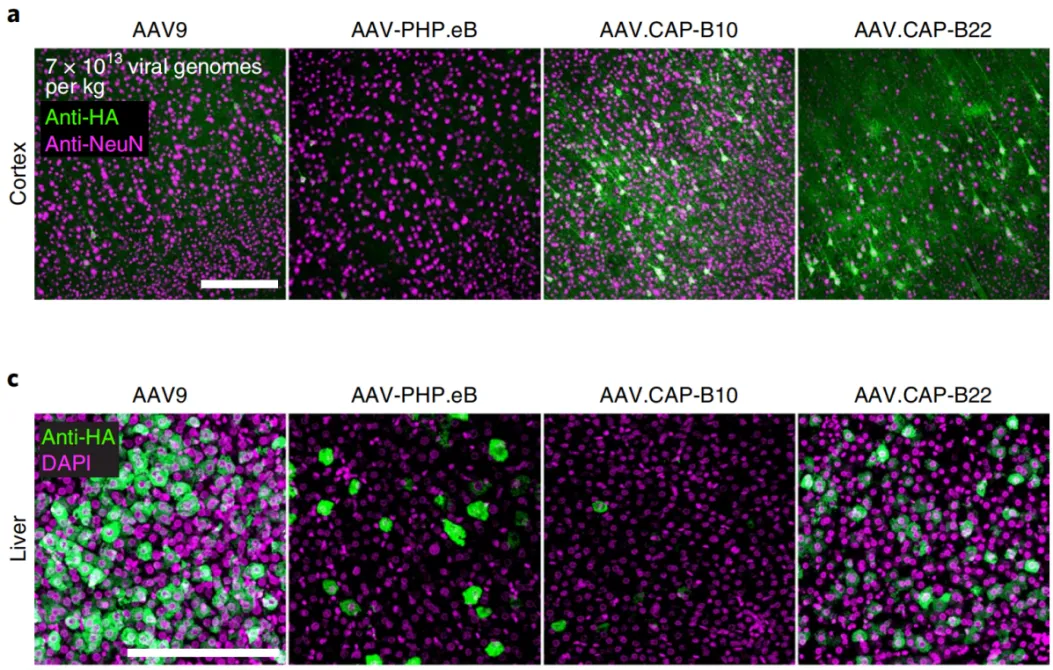 Figure 5:Transduction effects of AAV9, PHP.eB, CAP-B10, and CAP-B22 in marmosets
Figure 5:Transduction effects of AAV9, PHP.eB, CAP-B10, and CAP-B22 in marmosets
4. MaCPNS1 and MaCPNS2
On July 20, 2022, Viviana Gradinaru's team published a research paper titled "Engineered AAVs for non-invasive gene delivery to rodent and non-human primate nervous systems" in Neuron. To develop effective gene delivery vectors, the researchers performed directed evolution on the capsid of adeno-associated virus serotype 9 (AAV9) and identified two new capsid variants, MaCPNS1 and MaCPNS2. These new capsid variants were validated in rodents (mice and rats) as well as non-human primates (rhesus monkeys and marmosets).
Both AAV variants were shown to efficiently transduce the peripheral nervous system (PNS) of the experimental animals through intravenous injection. In non-human primates (NHPs), these AAV variants not only transduced the PNS but also the central nervous system (CNS). The researchers used intravenous injection to apply the AAV-MaCPNS1 vector in mice, which carried the jGCaMP8s and DREADD genes for monitoring ganglion calcium signals and chemogenetic control of dorsal root ganglion neurons, respectively.
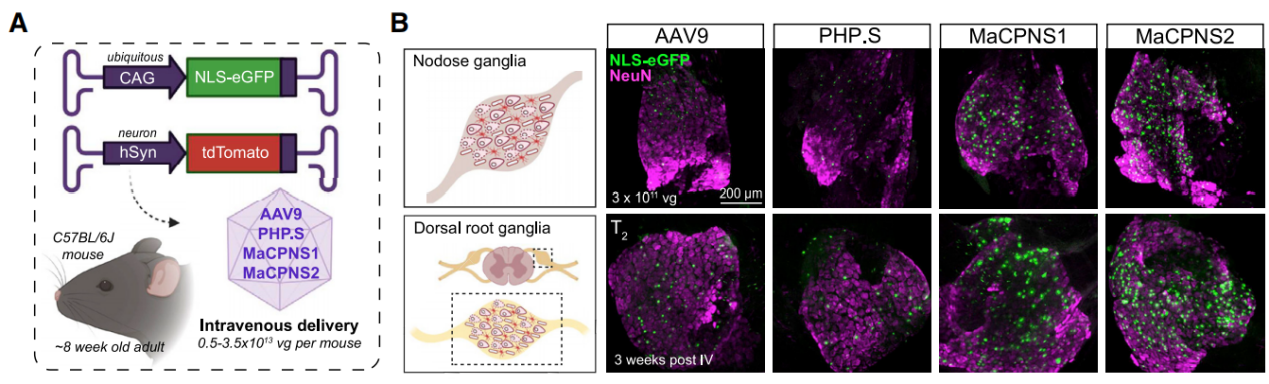 Figure 6:AAV9, PHP.S, MaCPNS1, and MaCPNS2 vector systems were used to mediate GFP expression through injection.
Figure 6:AAV9, PHP.S, MaCPNS1, and MaCPNS2 vector systems were used to mediate GFP expression through injection.
5. AAV.CPP.16
On October 10, 2022, Bei Fengfeng's team from Harvard Medical School published a research paper titled “Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood–brain barrier in rodents and primates” in Nature Biomedical Engineering. The study developed a virus vector, AAV.CPP.16, through rational modification and screening of the AAV9 capsid, which efficiently penetrates the blood-brain barrier in rodents and non-human primates (crab-eating macaques).
The screening revealed that AAV.CPP.16 not only has excellent blood-brain barrier penetration capabilities but also shows nearly an order of magnitude higher infection efficiency compared to AAV9. In cells infected with AAV.CPP.16, approximately 80% were neurons, around 15% were astrocytes, and about 1% were oligodendrocytes, indicating a high affinity of AAV.CPP.16 for central nervous system neurons. Additionally, AAV.CPP.16 proved to be an effective vector for delivering anti-tumor agents in a glioma mouse model.
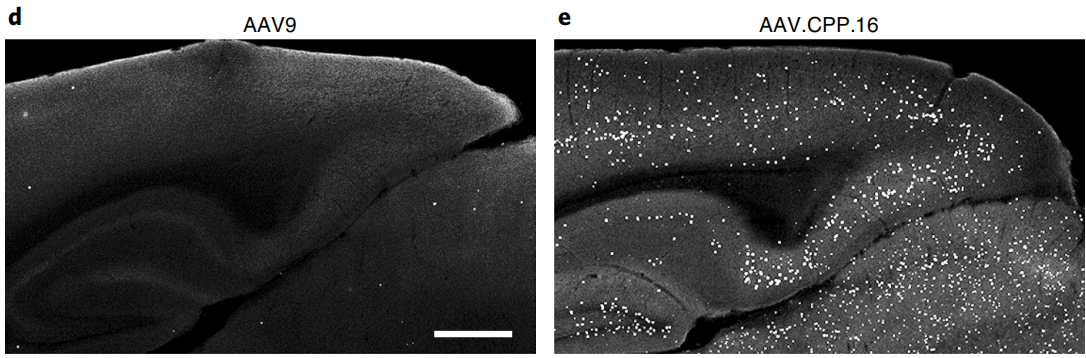 Figure 7:Transduction effects of AAV9 and AAV.CPP.16 in mice
Figure 7:Transduction effects of AAV9 and AAV.CPP.16 in mice
6. AAV.CAP-Mac
On July 10, 2023, Viviana Gradinaru's team published a research paper titled “Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain” in Nature Nanotechnology. They successfully developed a novel adeno-associated virus (AAV) variant, AAV.CAP-Mac, through directed evolution from AAV9, achieving efficient and non-invasive gene transfer in the brains of non-human primates (NHPs). AAV.CAP-Mac demonstrated excellent gene delivery efficiency in various primate species, including marmosets, rhesus macaques, and green monkeys.
In particular, AAV.CAP-Mac primarily targeted neurons in newborn Old World primates and predominantly targeted blood vessels in adult marmosets. The study also showcased the application of AAV.CAP-Mac in functional research, such as delivering functional GCaMP for in vivo calcium imaging and achieving Brainbow-like multicolor labeling in the macaque brain.
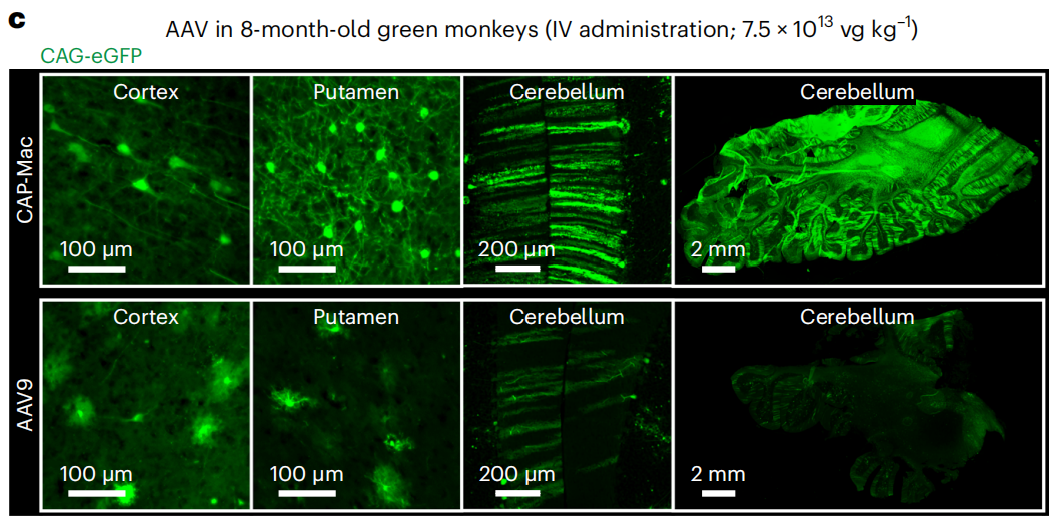
Figure 8:Intravenous injection of CAP-Mac into 8-month-old green monkeys mediates GFP expression.
7. AAV-BI-hTFR1
On May 16, 2024, the team led by Benjamin Deverman at the Broad Institute (with Huang Qin and Ken Y. Chan as first authors) published a research paper titled "An AAV capsid reprogrammed to bind human transferrin receptor mediates brain-wide gene delivery" in Science. The study designed an AAV capsid, BI-hTFR1, which binds to the human transferrin receptor (TfR1) expressed on the blood-brain barrier (BBB).
Compared to AAV9, BI-hTFR1 has a higher capacity for active transport across human brain endothelial cell layers and provides 40-50 times greater reporter gene expression in the CNS of mice with a human TFRC gene knock-in. This enhanced specificity is CNS-specific and does not occur in wild-type mice. When used to deliver the GBA1 gene (associated with Gaucher's disease and Parkinson's disease), BI-hTFR1 significantly increased glucocerebrosidase activity in the brain and cerebrospinal fluid, showing a notable improvement over AAV9. These findings provide scientific support for BI-hTFR1 as a potential vector for human CNS gene therapy.
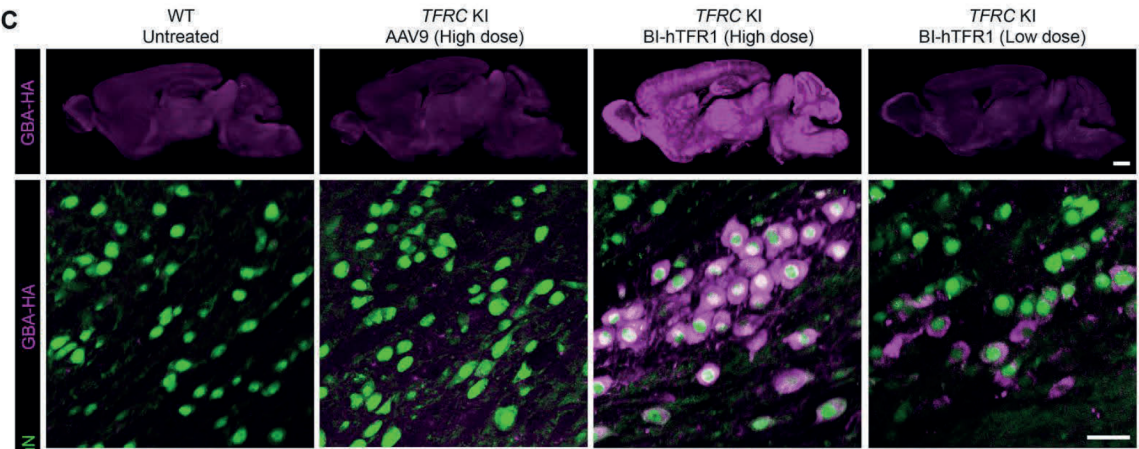 Figure 9:BI-hTFR1 efficiently delivers genes to TFRC knock-in mice
Figure 9:BI-hTFR1 efficiently delivers genes to TFRC knock-in mice
References:
[1]Deverman BE, Pravdo PL, Simpson BP, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016;34(2):204-209.
[2]Chan KY, Jang MJ, Yoo BB, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20(8):1172-1179.
[3]Goertsen D, Flytzanis NC, Goeden N, et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat Neurosci. 2022;25(1):106-115.
[4]Chen X, Ravindra Kumar S, Adams CD, et al. Engineered AAVs for non-invasive gene delivery to rodent and non-human primate nervous systems. Neuron. 2022;110(14):2242-2257.
[5]Yao Y, Wang J, Liu Y, et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood-brain barrier in rodents and primates. Nat Biomed Eng. 2022;6(11):1257-1271.
[6]Chuapoco MR, Flytzanis NC, Goeden N, et al. Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain. Nat Nanotechnol. 2023;18(10):1241-1251.
[7]Huang Q, Chan KY, Wu J, et al. An AAV capsid reprogrammed to bind human transferrin receptor mediates brain-wide gene delivery. Science. 2024;384(6701):1220-1227.
Brain Case offers packaging services for all the above serotypes(AAV-PHP.B, PHP.eB, PHP.S, CAP-B10, CAP-B22, MaCPNS1, MaCPNS2, CPP.16, CAP-Mac, BI-hTFR1). We have extensive experience in packaging these viruses, ensuring maximum yield and quality. If you have any questions, please don't hesitate to contact BD@ebraincase.com