- E-mail:BD@ebraincase.com
- Tel:+8618971215294
A fundamental goal of neuroscience research is to understand how the brain generates thoughts and actions. The brain is the most complex biological entity known to humans, making its study exceptionally challenging. One of the greatest obstacles to understanding the central nervous system is the heterogeneity of its molecules and cells—millions (in mice) to billions (in humans) of neurons densely packed and interconnected, interacting with each other and with non-neuronal cell types, including various types of glial cells and endothelial cells.
Over the past century or so, significant efforts have been made to better understand how the structure of the nervous system gives rise to its function. We have identified a tool capable of targeting neural system cells for infection—viral vectors. By carrying various fluorescent reporter genes or functional components (such as optogenetics and pharmacogenetics), we are able to exert long-term, specific, and high spatiotemporal resolution manipulation over certain cell populations within the central nervous system.
In the field of neuroscience research, neural circuits are regarded as the structural foundation for the coordination and execution of various basic and higher-order brain functions. Elucidating neural circuits is a prerequisite for understanding the principles of brain function and the pathogenesis of nervous system diseases.
Neurons integrate information received through dendritic spines (postsynaptic structures) from upstream neurons through axon terminals (presynaptic structures), and send signals to downstream neurons. The number of direct upstream and downstream partners for each neuron varies greatly from a few hundred to one hundred thousand, and neuronal information can be transmitted via convergent or divergent neural pathways through monosynaptic or multisynaptic connections.
Can we utilize a specific class of labeling and tracing tools to elucidate the connectivity within these neural circuits? Subsequently, we will introduce several commonly used methods for investigating an important topic in neuroscience research: the output circuits of anterograde monosynaptic tracing work.
In 2017, Professor Li Zhang’s team at the University of Southern California published an article in the journal Neuron, confirming for the first time that serotype 1 of adeno-associated virus (AAV/1) can achieve anterograde trans-monosynaptic tracing. Infection of presynaptic neurons with high titers of AAV1-Cre/Flp can effectively drive the expression of Cre/Flp-dependent exogenous genes in postsynaptic neurons in a specific manner across a single synapse, thereby enabling anterograde monosynaptic transmission and labeling of neurons by AAV1. However, due to the high titer required for AAV1 infection and the low efficiency of synaptic transmission, it is usually necessary to introduce the Cre/lox or Flp/frt system, combined with Cre/Flp-dependent fluorescent reporter AAVs (such as AAV-DIO/FLEx/fDIO-EGFP), to amplify the signal of fluorescence in downstream neurons. Moreover, due to the specificity of high-titer AAV1 transduction mechanism, it is unable to achieve upstream cell-specific anterograde trans-monosynaptic tracing.
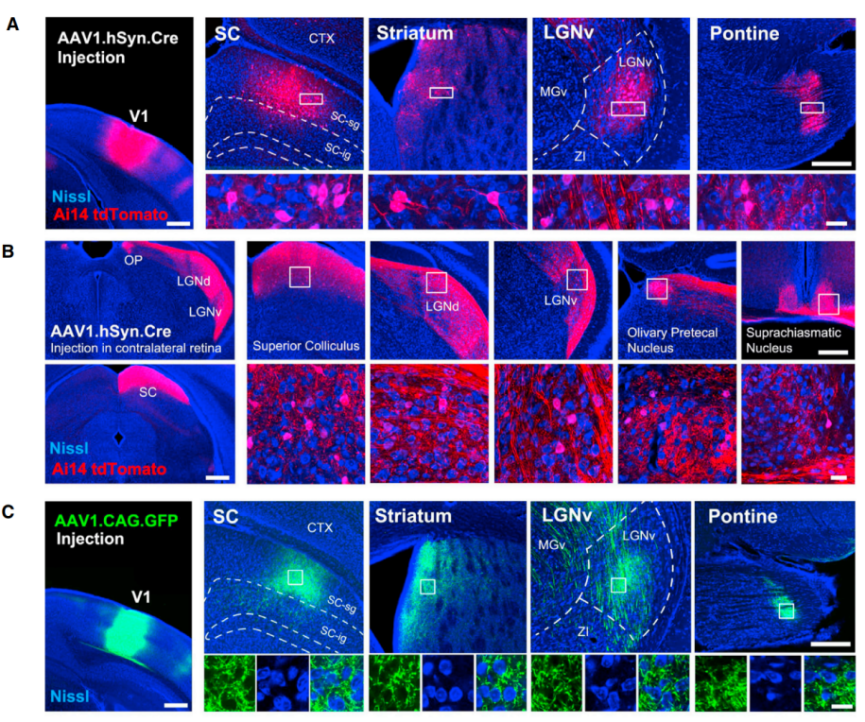
Figure 1:AAV1 anterograde trans-monosynaptic tracing system
(Source: AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. DOI:https://doi.org/10.1016/j.neuron.2016.11.045)
| Viral Vector Backbone | Titer | Serotype | Remark |
| rAAV-hSyn/CAG-cre | ≥1.0e+13 VG/mL | AAV2/1 | carries a fluorescent protein and has low efficiency in mono-synaptic anterograde tracing, but introducing the Cre/lox system can increase the efficiency of synaptic transmission. AAV1 is not compatible with Cre-driver line-based circuit tracing. |
On May 6th 2022, the laboratory of Professor Xin Duan at the University of California, San Francisco (UCSF) published an article in Nature Neuroscience and developed a new set of single-cell sequencing technology based on transsynaptic tracing by using genetic engineering to modify wheat germ agglutinin (WGA) and screening a specific fusion protein product mWGA-mCherry (mWmC), and infecting specific target cell types with an adeno-associated virus vector (AAV), it can achieve monosynaptic anterograde tracing.
Researchers synthesized a mammalian codon-optimized cDNA (mWGA) to enhance WGA expression in mammalian neurons. This feature highlights their optimization efforts directed towards WGA expression in neurons to improve experimental efficacy and result reliability.
To maximize compatibility with existing green fluorescent protein (GFP) lines for retinal ganglion cell types and identify efficient fluorescent protein fusion configurations, they generated mWGA constructs fused to red fluorescent protein (RFP) variants, including mCherry, mRuby3, and tdTomato. This feature underscores their consideration of compatibility with existing data and methods in experimental design, as well as their active exploration of different fluorescent protein fusion schemes to achieve optimal experimental outcomes.
Finally, the mWmC configuration possesses a lower level of retrograde transduction efficiency, thereby enhancing the accuracy of anterograde monosynaptic tracing studies.
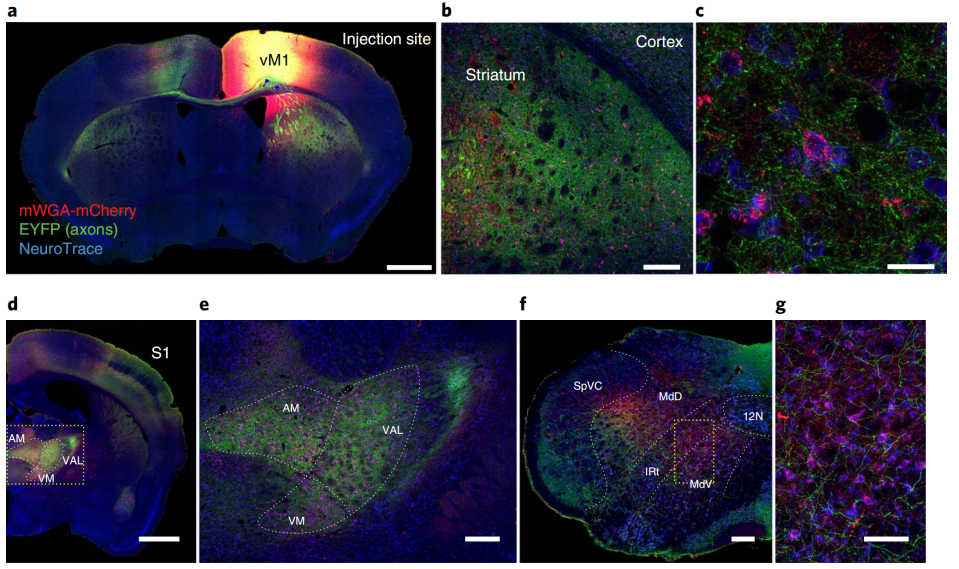
Figure 2: Anterograde trans-monosynaptic tracing of M1 brain region by using mWmc system
(Source:Trans-Seq maps a selective mammalian retinotectal synapse instructed by Nephronectin. https://doi.org/10.1038/s41593-022-01068-8 )
| Viral vector backbone | Titer | Serotype | Remark |
| rAAV -CAG-mWGA-mCherry-WPRE | 2.00e+13 VG/mL | AAV2/2 | Similar to secreted proteins, it is normal for labeled cells to have unclear outlines. |
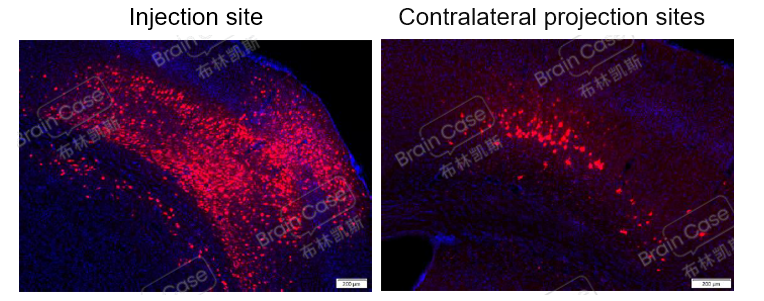
Figure 3: After codon optimization, mWGA-mediated anterograde AAV virus injection in the M1 brain region of C57BL/6 mice showed significant red fluorescence expression at the injection site and downstream brain areas on the contralateral side 21 days post-injection, indicating that the virus can be used for anterograde tracing experiments.
| Product Name | rAAV-CAG-DIO-mWGA-mCherry | rAAV-hSyn-SV40 NLS-Cre |
| Serotype | 2/9 | 2/9 |
| Product ID | BC-1227 | BC-0159 |
| Product Titer | 5.04×1012 vg/mL | 3.12×1012 vg/mL |
| Test Animal | C57BL/6 | C57BL/6 |
| Injection Volume | 300 nL/animal(Pre-mixed) | |
| Test Site | M1, AP=+1.54mm,ML=+1.60mm,DV=-1.60mm | |
The research team led by Professor Xu Fuqiang at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, developed a rigorous and practical monosynaptic anterograde tracing recombinant virus H129 system. They embedded the anti-Her2 scFv (single-chain antibody variable) region into the gDΔ6–38 region of the H129 envelope glycoprotein D (gD) to infect neurons that only express the Her2 receptor. This achieved specificity in targeting the initial site of H129 to Her2-expressing cells, thereby eliminating the reuptake ability of the original H129 strain at axon terminals.
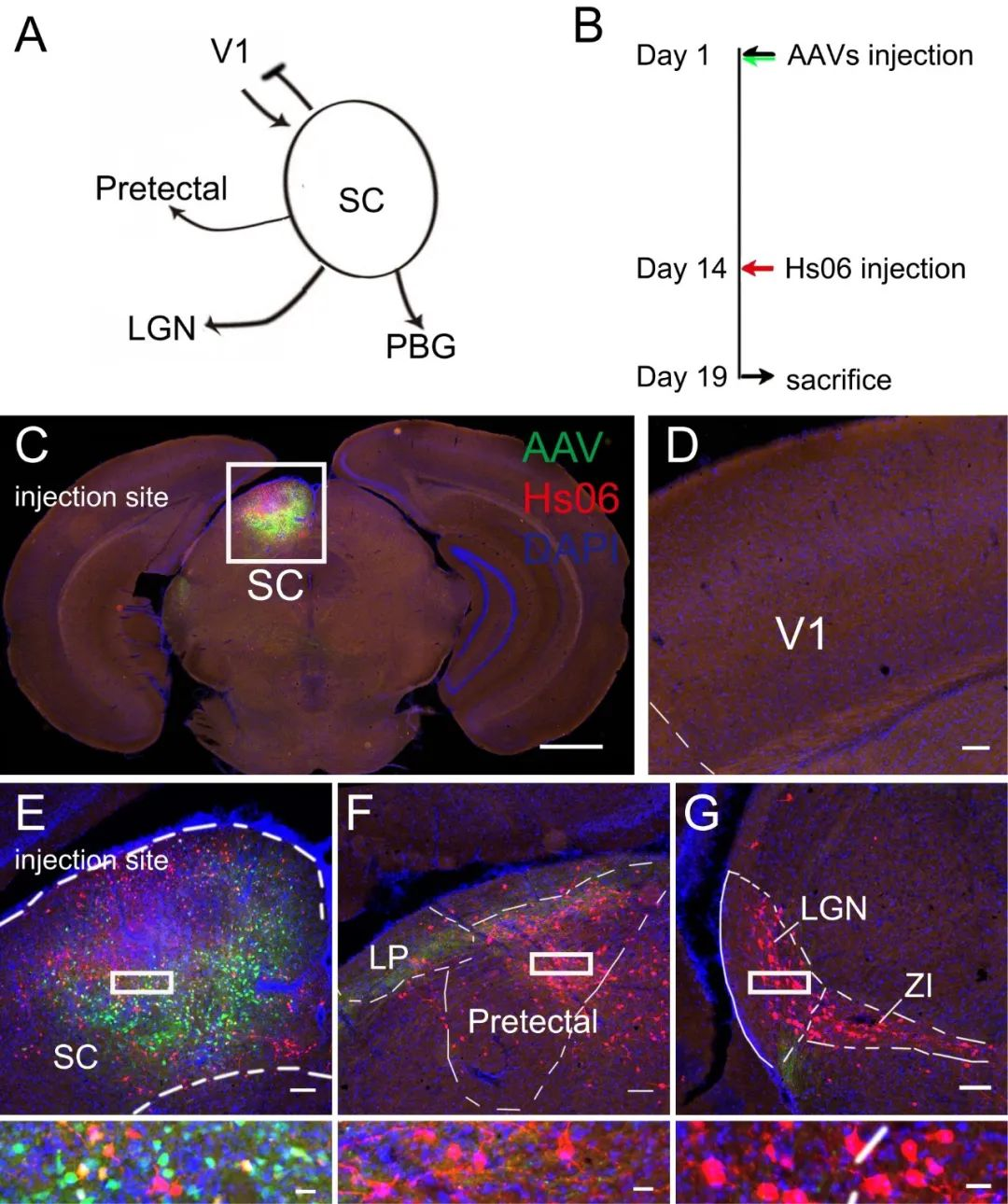
Figure 4: Using the Hs06 anterograde trans-monosynaptic labeling and tracing system to study the next-level circuits of SC
(Source:Rigorous anterograde trans-monosynaptic tracing of genetic defined neurons with retargeted HSV1 H129, doi: https://doi.org/10.1101/2020.12.01.407312)
To confirm Hs06 virus system could realize anterograde trans-monosynaptic tracing without retrograde spreading, they performed tracing experiment initial form superior colliculus (SC) (Fig.4). It had been proved that SC only received projection form V1 but not projected back. Neurons in SC project to many regions including pretectal nucleus and LGN. In this experiment, they observed neurons labeled by Hs06 in SC projecting regions but not V1 (Fig.4C-G). These results suggested that the system could anterograde trans-monosynaptic spread exclusively with the assistance of Her2CT9 and gD.
| Product No. | Backbone | Remark |
| BC-1245 | rAAV-hSyn-DIO-EGFP-T2A-Her2CT9 | Especially suitable for cell-specific anterograde trans-monosynaptic tracing output networks of the upstream starting injection site. |
| BC-1356 | rAAV-UL26.5-DIO-cmgD | |
| BC-HSV-Hs06 | H129-dgD-hUbC-mCherry-P2A-scHer2::gd |
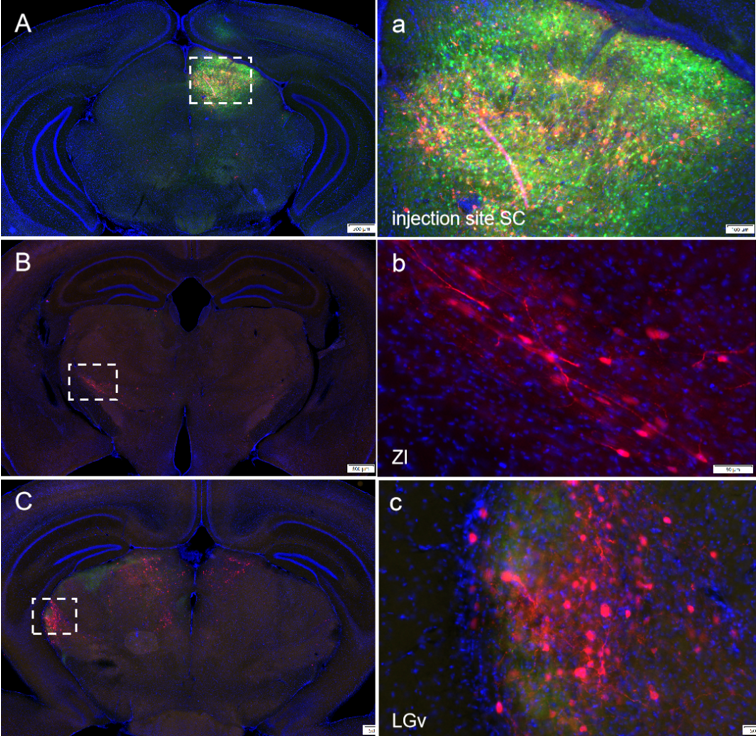
Figure 5: The mixing ratio of AAV auxiliary viruses BC-0159:BC-1245:BC-1356=1:2:8 (Cre : Her2 : gD), volume 100 nl, was injected into the SC brain region. After 14 days of expression, HSV was injected at the same site, and the animals were further maintained for 5 days before perfusion and tissue collection. Fixed slices were imaged. The results showed neurons labeled with red fluorescence by HSV in downstream brain regions of the injection site, achieving the HSV anterograde trans-synaptic strategy.
| Product Name | H129-dgD-ubc-mCherry-p2A-scher2::gd | rAAV-UL26.5-DIO-cmgD | rAAV-hSyn-DIO-EGFP-T2A-Her2CT9 |
| Serotype | / | 2/9 | 2/9 |
| Product ID | BC-HSV-Hs06 | BC-1356 | BC-1245 |
| Product Titer | 1.00×108 PFU/mL | 5.31×1012 vg/mL | 3.00×1012 vg/mL |
| Test Animal | C57BL/6 | C57BL/6 | C57BL/6 |
| Injection Volume | 300 nL/animal(Pre-mixed) | ||
| Test Site | SC, AP=-3.64mm,ML=-1.30mm,DV=-2.35mm | ||
The following are the neural circuit tracing experimental services that Brain Case can provide, which include but are not limited to the following demonstrations:
| Serial No. | Experimental project type | Service |
| 1 | rat/mouse PRV retrograde muti-synaptic tracing (PNS) |
|
| 2 | rat/mouse PRV retrograde muti-synaptic tracing (CNS) | |
| 3 | rat/mouse HSV anterograde muti-synaptic tracing (CNS) | |
| 4 | mouse HSV anterograde mono-synaptic tracing (CNS) | |
| 5 | at/mouse RV retrograde mono-synaptic tracing (CNS) | |
| 6 | rat/mouse RV retrograde three-level neural circuit tracing(CNS) |
Injection site or target brain area coordinates, imaging brain area location, etc. Among them, the three-level projection of A→B→C requires confirmation that there is a projection relationship between B and C.
Experimental plan → Evaluation → Plan adjustment → Experimental implementation → Data control → Feedback/analysis
If you are interested in the details of the experiment or possible problems and causes during the experiment, please contact: BD@ebraincase.com