- E-mail:BD@ebraincase.com
- Tel:+8618971215294
AAV is one of the most commonly used viral vectors in the field of gene therapy. In recent years, AAV gene therapy has made progress in a variety of indications, including rare eye diseases, hemophilia, rare muscle diseases, central nervous system diseases, lysosomes Body storage disease, etc. As a small non-enveloped cellular virus, AAV has many advantages in delivery systems, such as non-pathogenicity, efficient and sustained expression, easy operation and low immunogenicity. Due to biological differences between species, adverse effects following systemic administration have not been observed in mice or preclinical animal models. Therefore, improving viral vectors to cope with differences in AAV translation across species mitigates potential risks, improves the therapeutic window associated with AAV gene therapy, and enables better predictive modeling, leading to effective clinical translation.
Aravind Asokan's team at Duke University School of Medicine published an article "Cross-species evolution of a highly potent AAV variant for therapeutic gene transfer and genome editing" in "Nature Communications" and developed an efficient, cross-species compatible AAV variant ( AAV.cc47), experimental results show that in normal mice and diseased mouse models, compared with AAV9, the mutant AAV.cc47 treatment group has higher reporter gene and therapeutic gene expression, and Cre recombination and CRISPR genome editing efficiencies are also higher. higher. The enhanced transduction efficiency of AAV.cc47 vector was further confirmed in macaques and pigs, providing a strong theoretical basis for potential clinical translation and human gene therapy.

In normal mice, comparing the transduction efficiency of AAV.cc47 and AAV9, the results showed that the transduction efficiency of AAV.cc47 increased in the heart, tibialis anterior muscle and brain, and the increase in transduction efficiency of AAV.cc47 in the liver was statistically significant. (Fig. 2b,c). Quantitative analysis of the fluorescence intensity of the heart, tibialis anterior muscle, and liver showed that the fluorescence intensity of AAV.cc47 was 21 times, 16 times, and 2 times higher, respectively (Figure 2d). Compared with AAV9, AAV.cc47 transduced more neurons in the cortex (CTX), cerebellum (CB) and hippocampus (HC), and the transduction amount increased by 4 times, 3 times and 4 times respectively. The transduction efficiency of AAV.cc47 was increased in the heart, tibialis anterior muscle, and brain of Ai9 mice, while there was no significant difference in transduction efficiency in the liver. Studies have shown that there is no difference in the biodistribution of AAV.cc47 and AAV9 in various tissues (Figure 3e), while the number of vector genome copies in tissues is vector dose-dependent.
In addition, studies have shown that AAV.cc47 is more selective in targeting neuronal cells in the brain. The genome editing efficiency of two AAV serotypes was tested in Ai9 mice, and the results showed that AAV.cc47-CRISPR genome editing efficiency was higher in heart and skeletal muscle. Interestingly, compared with AAV9, the expression of gRNA in three tissues of mice in the AAV.cc47 treatment group increased 3-4 times.
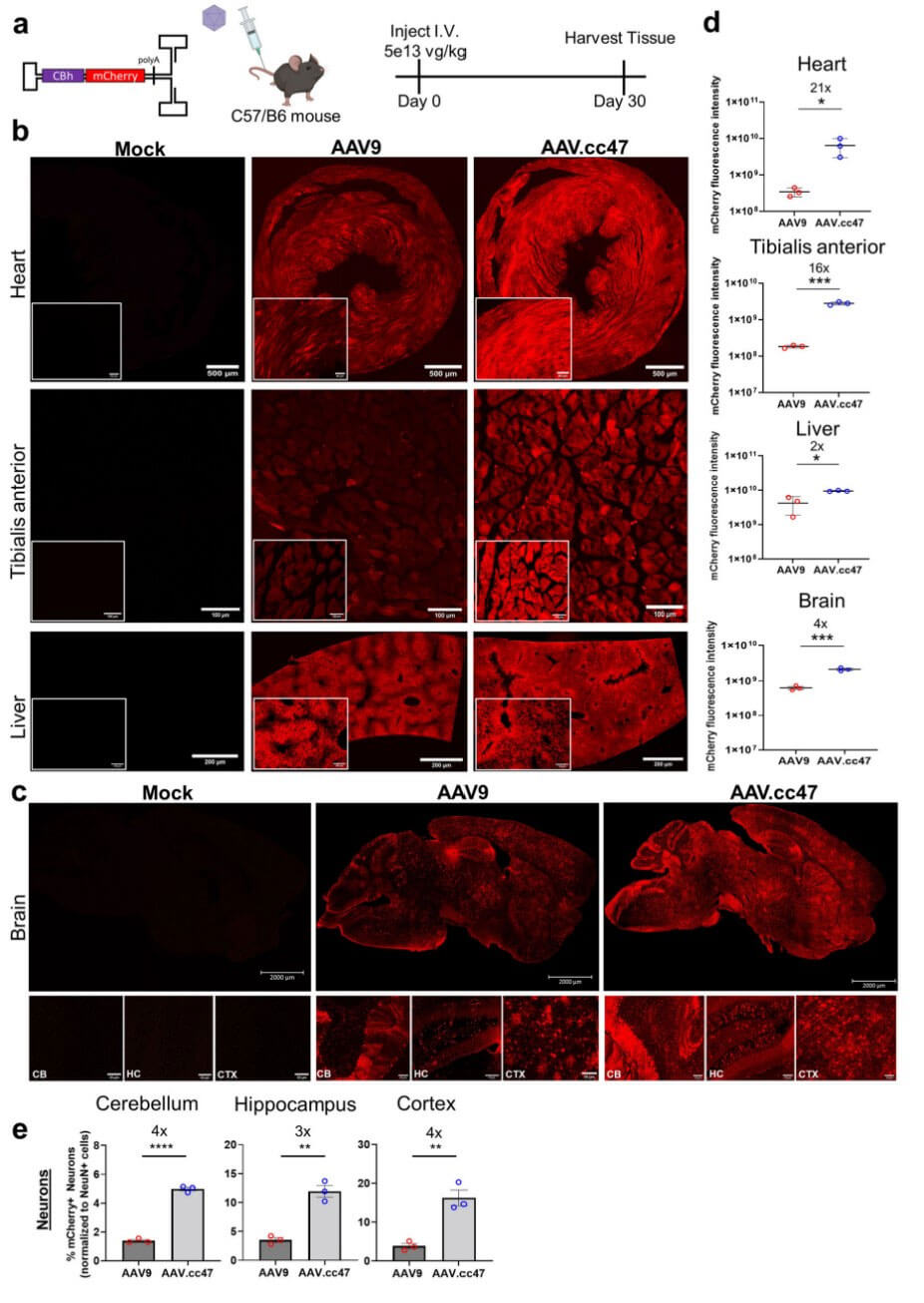
Comparing the transduction efficiency of AAV.cc47 and AAV9 in non-human primate cynomolgus monkeys by intracerebroventricular injection, the results showed that AAV.cc47 expression and diffusion ability were stronger in the cerebral cortex and cerebellum of monkeys, while AAV was stronger in the cerebral cortex. .cc47 exhibits higher penetration ability. Compared with AAV9, AAV.cc47 has higher transduction efficiency into cortical neurons, glia, and Purkinje cells. To evaluate the transduction efficiency of AAV.cc47 in other species, the researchers tested it on neonatal mice and pigs. The results showed that AAV.cc47 is a cross-species compatible vector and can achieve transduction in the brains of mice, pigs and NHPs. Higher transduction efficiency.
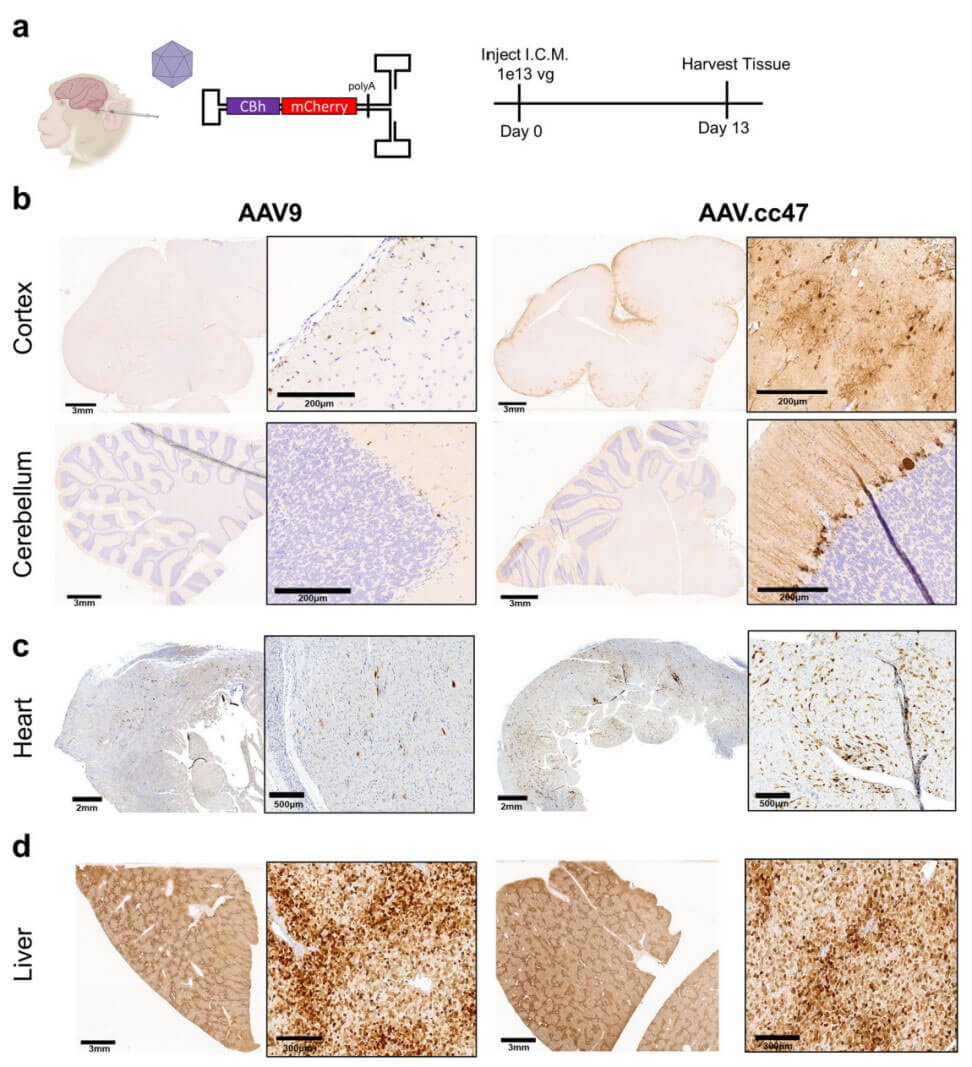
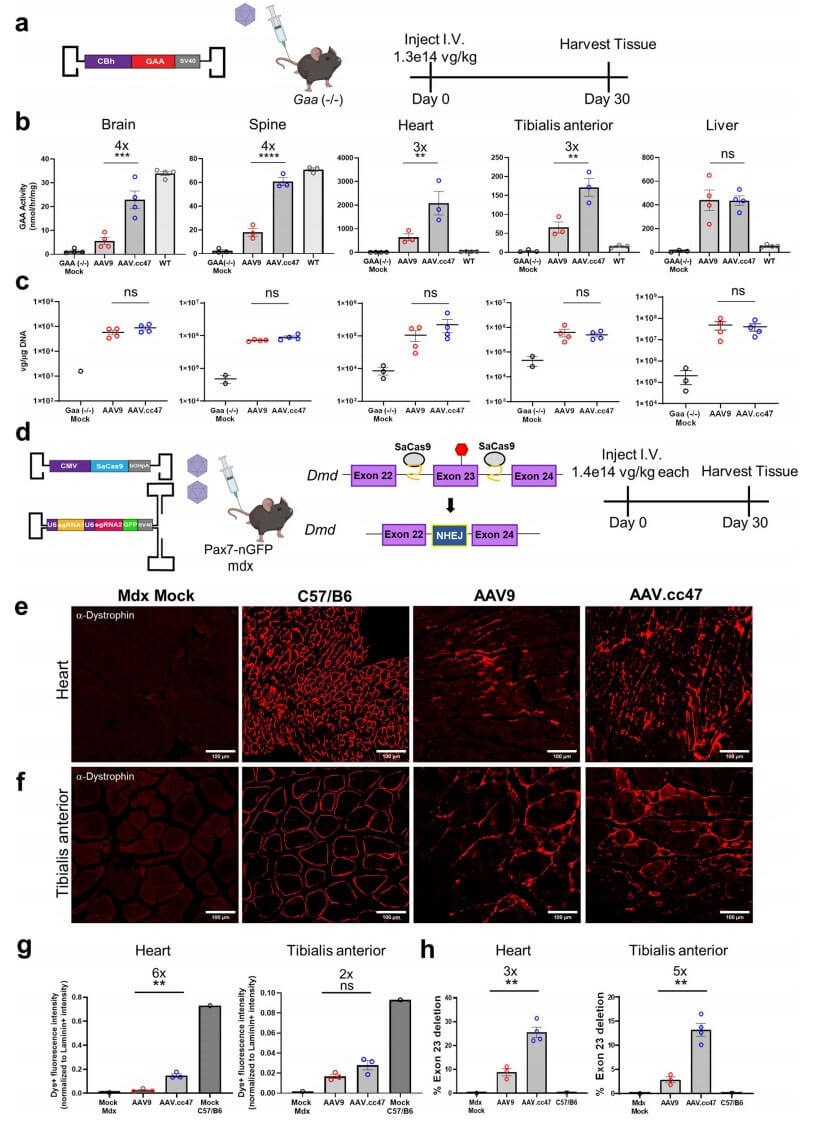
Pompe disease is a rare disease, also known as acid alpha-glucosidase deficiency or glycogen storage disease type II (GSD II). Due to the lack of acid α-glucosidase (GAA) in lysosomes, glycogen and maltose cannot be converted into glucose and utilized. As a result, a large amount of glycogen in the body accumulates in tissue cells such as skeletal muscle, cardiac muscle, and smooth muscle, causing disease. In order to explore the ability of AAV.cc47 vector to deliver therapeutic genes in mouse disease models, the researchers compared the delivery capabilities of AAV.cc47 and AAV9 vectors in Pompe disease (Gaa−/−) mouse models. Analysis of GAA enzyme levels in mouse brain tissue 4 weeks after administration showed that GAA expression in the AAV.cc47-GAA treated group was higher, and the enzyme activity level reached 67% of that of WT mice, while in the AAV9-GAA treated group only 16% of WT levels in mice. In the mouse heart and tibialis anterior muscle, only the AAV.cc47-GAA treatment group showed a significant increase in enzyme activity.
Duchenne muscular dystrophy is a progressive skeletal muscle weakening disease caused by mutations in the DMD gene encoding dystrophin on the X chromosome. In mdx mouse hearts, dystrophin expression was 6-fold higher in the AAV.cc47-CRISPR-treated group than in the AAV9 group. In the tibialis anterior muscle of mdx mice, the dystrophin recovery rate in the AAV.cc47-CRISPR treated group was 2 times higher than that in the AAV9 group. Quantitative results showed that compared with AAV9, AAV.cc47-mediated genome editing increased transcripts with exon 23 deletions in the heart and tibialis anterior muscle by 3-fold and 5-fold, respectively.
This study demonstrates that cross-species evolution can produce highly efficient AAV variants capable of transducing multiple tissues in different preclinical animal models. Evolving a capsid library via VR-IV residue saturation mutagenesis significantly improved the transduction profile of AAV9 in three different animal models. This cross-species approach is suitable for the evolution and screening of AAV libraries and, when combined with promoter-specific selection strategies, can improve the clinical translatability of next-generation AAV vectors.
The following interpretation only represents Xiaobu's personal understanding and opinion of the article. All the pictures used in this interpretation are from the original text. If you need more information, please refer to the original literature