- E-mail:BD@ebraincase.com
- Tel:+8618971215294
On March 2, 2023, the Karl Deisseroth research team from Stanford University published an article in the top international journal "Nature". In this study, they confirmed that increased heart rate can cause anxiety-related behaviors in mice, proving that from the body How signals influence affective behaviors related to emotions such as anxiety and fear.
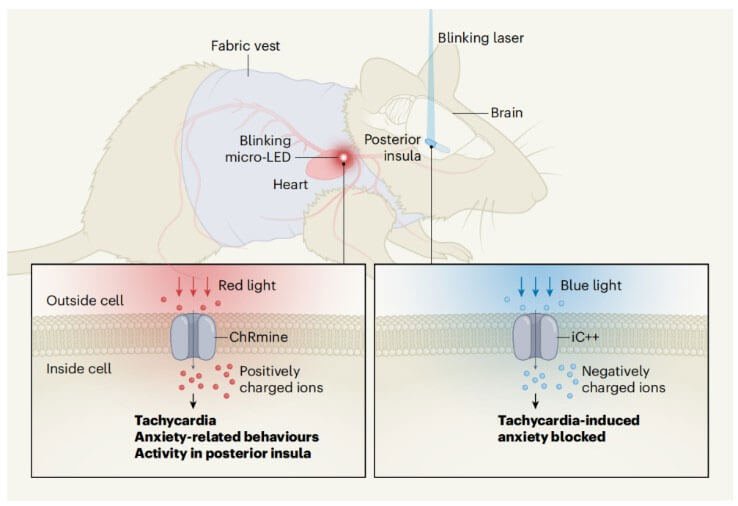
Optogenetics can make target cells express light-sensitive proteins, and use light as a tool to control the switch of cell functions, achieving precise control of individual nerve cells in milliseconds. It can be described as an explosive black technology in neuroscience research. In this study, the Deisseroth team used a rhodopsin protein called ChRmine, which has high sensitivity and the ability to recognize red. Characteristics of light, these two characteristics mean that the use of low irradiation intensity and strong penetrating red light can help us achieve non-invasive impact on deep tissues.
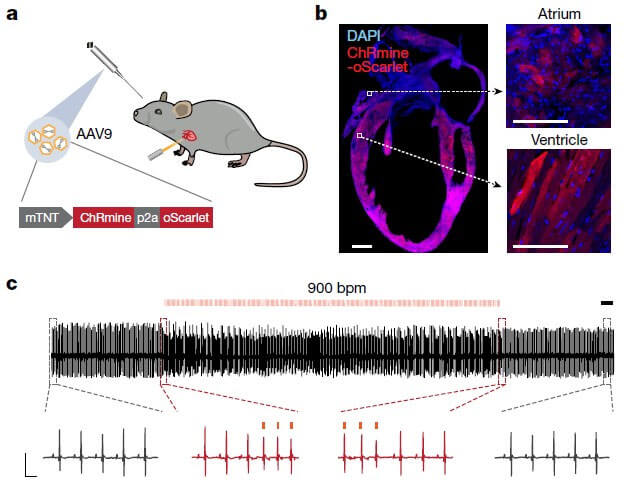
The mouse cardiac troponin T promoter (mTNT) is connected to ChRmine, AAV9 is used to mediate gene expression, and ChRmine can be specifically expressed in the mouse myocardium by injecting it through the retroorbital vein of mice. The results show that 589nm illumination of the chest of anesthetized mice can significantly increase the heart rate of the mice, and the normal pacing state can be restored after stopping the illumination. In vitro cardiomyocyte experiments also verified the feasibility of the system.
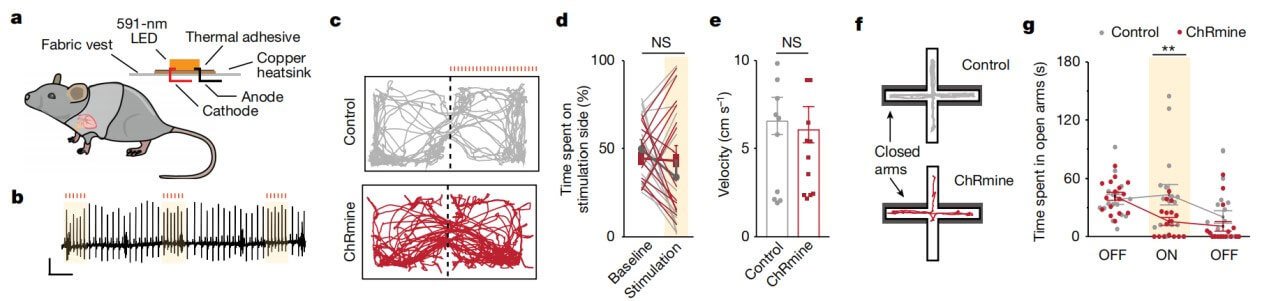
In order to facilitate the experiment and not affect the normal behavior of the mice, the caring and crazy researchers also made a special vest for the mice. The vest is equipped with a micro-LED that emits 591nm light. The LED light penetrates the chest wall of the mouse and reaches the heart, activating the entire system and increasing the mouse's heart rate. Using this device, researchers were able to increase the heart rate of mice from the normal 660 beats per minute to 900. Mice with accelerated heartbeats showed obvious behavioral signs of anxiety.
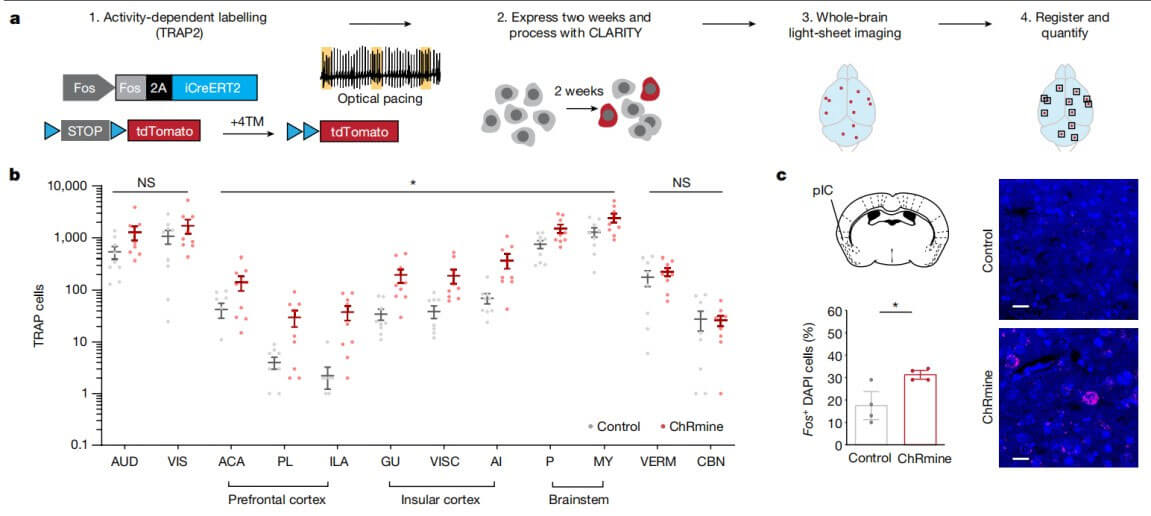
To further investigate the potential link between cardiac tachycardia and brain behavioral regulation, the research team used the characteristics of TRAP2 transgenic mice to screen regions throughout the brain that are activated in cardiac tachycardia. These areas include areas related to the central autonomic neural network, such as the prefrontal cortex, insular cortex, and brainstem. Many other cortical areas involved in autonomic or interoceptive processing were not significantly activated, including primary sensory auditory (AUD) and visual (VIS) cortical areas, among others. Consistent with the TRAP2 mapping results, optical pacing also increased endogenous expression of Fos mRNA in the posterior insular cortex (pIC).
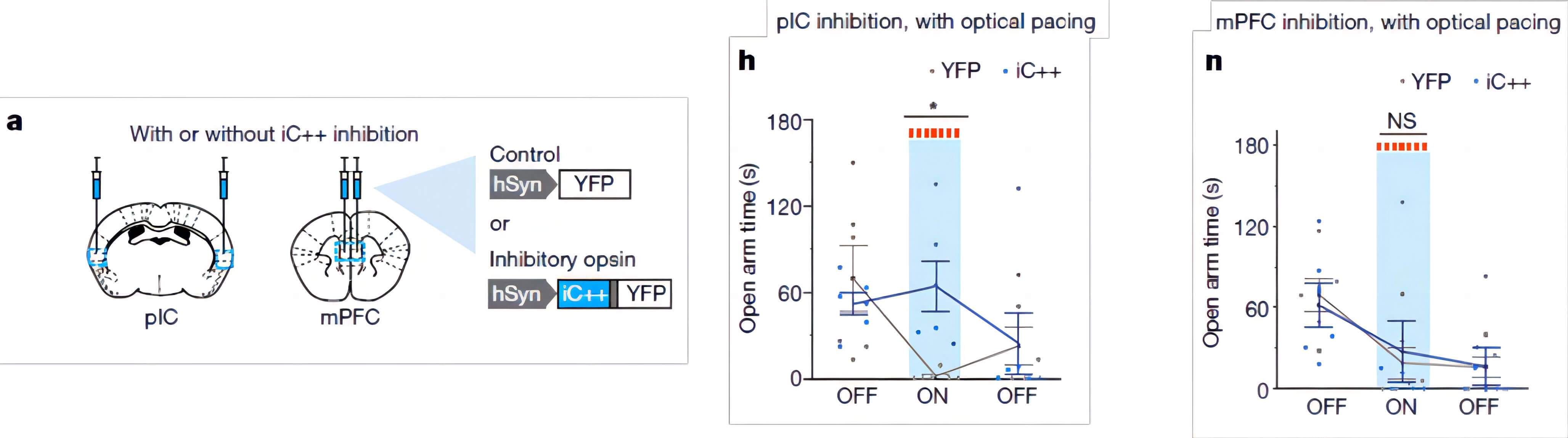
The insula is a well-established processing center for emotional and physical signals. To determine whether anxiety in mice caused by tachycardia was specifically modulated by neurons in the pIC region, the researchers used another rhodopsin, iC++, to inhibit the posterior insula. Activity of neurons in the cortex
By using optogenetic inhibition with 473nm blue light, the researchers found that inhibiting the activity of neurons in the posterior insular cortex using rhodopsin iC++ significantly improved anxious behavior caused by tachycardia during optical pacing. This inhibitory phenomenon is specific to the posterior insular cortex, as it was not observed in optogenetic inhibition of another region, the medial prefrontal cortex (mPFC).
To summarize, the team of Karl Deisseroth, the father of optogenetics from Stanford University, used a non-invasive optogenetic "pacemaker" to give mice a heartbeat adventure and found that tachycardia can effectively enhance anxiety-like behaviors. Suppressing activity in the brain's posterior insular cortex can attenuate this effect. This discovery shows that both the brain and body may be involved in the development of emotions, and it also means that we may be able to treat problems such as anxiety by intervening in the heart.
[1]: Hsueh B, Chen R, Jo YJ, et al. Cardiogenic control of affective behavioral state[J]. Nature, 2023: 1-8.
[2]: Allen WE, DeNardo LA, Chen MZ, et al. Thirst-associated preoptic neurons encode an aversive motivational drive[J]. Science, 2017, 357(6356): 1149-1155.
The following interpretation only represents Xiaobu's personal understanding and opinion of the article. All the pictures used in this interpretation are from the original text. If you need more information, please refer to the original literature