- E-mail:BD@ebraincase.com
- Tel:+8618971215294
In recent years, increasing attention has been directed toward the potential role of the cerebellum in higher functions beyond sensorimotor coordination, such as emotional and cognitive processing. The limbic system serves as the central hub for emotion regulation, with the amygdala playing a key role in the bidirectional regulation of anxiety. Motor coordination and emotional control are two independent yet interconnected functions of the central nervous system (CNS). Motor activity not only constitutes an essential component and physiological basis of emotional expression but also improves mental health by alleviating negative emotions and enhancing cognitive function. Indeed, multiple prospective cohort studies support the protective effects of physical exercise against anxiety and depression. However, the neural basis underlying the interaction between motor control and emotional processing remains poorly understood. In this article, the researchers explore a hypothalamo-cerebello-amygdalar circuit that may mediate exercise-dependent anxiety alleviation.
Anxiety disorders often co-occur with other psychiatric conditions. Researchers collected resting-state functional magnetic resonance imaging (fMRI) data from patients with bipolar disorder (BD), where anxiety is the most common comorbid diagnosis. Compared to healthy controls (HC), the functional connectivity between the cerebellar nuclei and the amygdala was found to be weaker on average in bipolar disorder patients (Figure 1A). This weaker correlation suggests a potential interaction between these two brain regions in BD patients.
The researchers injected the viral tracer AAV2/9-hSyn-mCherry into the cerebellar nuclei of rats (Figure 1E; see Figure S1A in the original text for details). mCherry-positive afferent fibers were predominantly distributed in the centrolateral zone (CeL) of the amygdala. When they injected the retrograde tracer AAV2/2-Retro-eGFP into the CeL (Figures 1G and S1C), they found that the cerebellar neurons projecting to the amygdala were primarily concentrated in the dentate nucleus (DN), the phylogenetically youngest cerebellar nucleus, which has been increasingly associated with emotional regulation.
To structurally validate these projections, the researchers employed monosynaptic retrograde rabies virus tracing and fluorescence micro-optical sectioning tomography (fMOST) to map the long-range direct projections from DN neurons to CeL neurons in mice at a mesoscale level (Figure S2A).

Figure 1. A direct cerebello-amygdalar circuit negatively correlated with anxiety
Researchers subjected rats to continuous running on a rotating rod at a constant speed of 10 rpm for four consecutive days. Compared to their non-exercising littermate controls, these rats showed a greater preference for the open arms of the elevated plus maze and the illuminated area of the light-dark box, while their total movement distance remained unaffected (Figures 2A and 2B). These data suggest that exercise induced a reduction in anxiety levels.
By recording the Ca²⁺ activity of CeL neurons during rotating rod running, it was found that 73.3% (22/30) of the recorded CeL neurons were activated during the exercise. This activation rate was comparable to the proportion of CeL neurons (66.6%, 20/30) activated by optogenetic stimulation of the dentate nucleus (DN) (Figures 2D–2G). Notably, the majority of neurons (56.6%) responded to both the rotating rod running and optogenetic stimulation (Figures 2D–2G; Video S2).
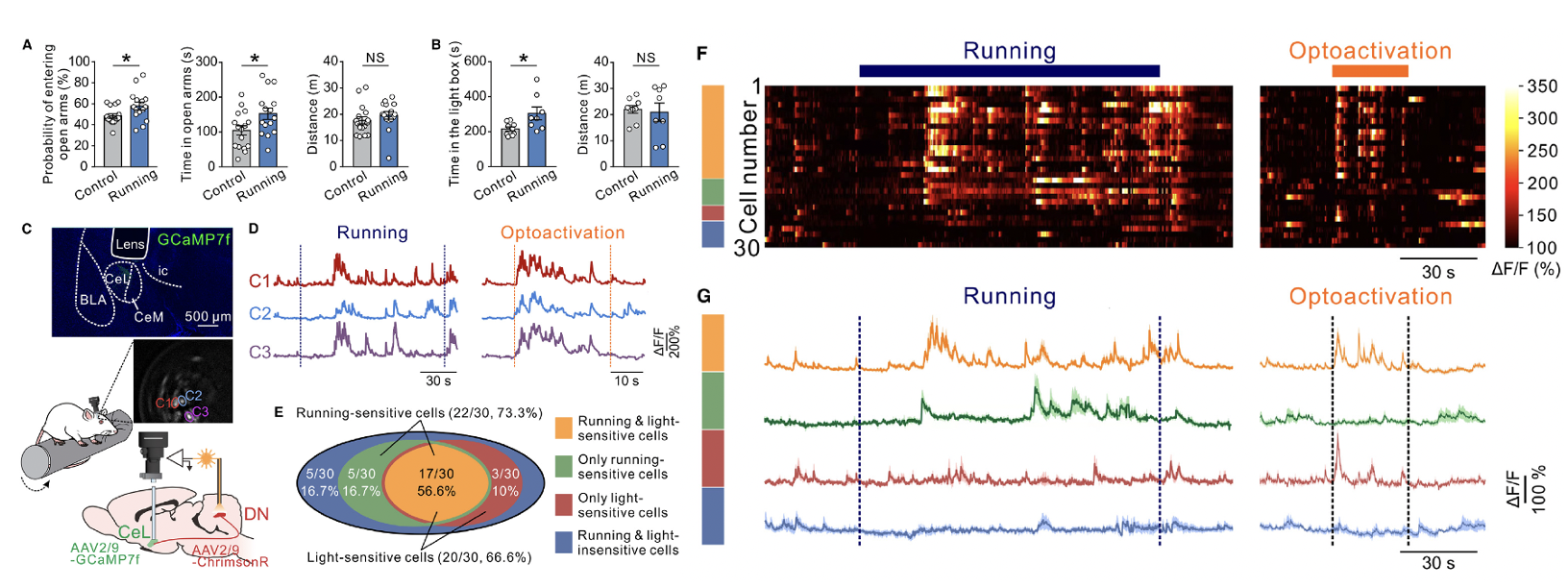
Figure 2. Rotarod running produces anxiolytic-like behaviors and activates CeL neurons receiving direct inputs from cerebellar DN
To verify that the activation of CeL neurons during rotating rod running was triggered by cerebellar afferent input, researchers optogenetically inhibited the DN-CeL pathway. This inhibition resulted in a significant reduction in Ca²⁺ activity of CeL neurons (Figures S3C–S3G; Video S3). The proportion of movement-sensitive neurons activated by optogenetic stimulation of the DN was consistent with these findings. These results indicate that the DN-CeL projection conveys exercise-dependent information.
fMRI showed that after 4 days of running on a rotating rod at a constant speed of 10 rpm, functional connectivity between the cerebellar nuclei and the amygdala increased (Figures 3A–3C), suggesting that exercise may induce plasticity in the cerebellar-amygdalar circuit. At the electrophysiological level, light stimulation of DN afferent fibers expressing ChR2 elicited fast excitatory postsynaptic currents (EPSCs) in CeL neurons, with an average latency of 3.73 ± 0.24 milliseconds (Figure 3F). The optogenetically evoked EPSCs could be blocked by tetrodotoxin and restored with the addition of 4-aminopyridine (Figure 3G), indicating that glutamatergic inputs from DN fibers to CeL neurons exhibit a monosynaptic nature.
Hypothesizing that DN afferent activity might induce long-term synaptic plasticity in CeL neurons, researchers injected AAV-hSyn-oChIEF into the DN and applied high-frequency optogenetic stimulation to oChIEF-expressing afferent terminals in the CeL. This stimulation induced long-term potentiation (LTP) of EPSCs at DN-CeL synapses. Notably, the CeL neurons with optogenetically evoked EPSCs and LTP were all positive for PKCδ (Figure 3J). Furthermore, a Cre-dependent trans-synaptic retrograde tracing system (Figures S5C–S5F) confirmed that DN glutamatergic neurons directly form monosynaptic projections onto PKCδ+ neurons in the CeL.
To investigate whether glutamatergic inputs from DN to CeL influence anxiety-like behaviors, researchers injected AAV-CaMKII-ChR2 into the DN to optogenetically activate DN glutamatergic projections targeting terminals in the CeL (Figure 3K). This activation significantly improved anxiety-like behaviors in the elevated plus maze (Figure 3L) and concurrently increased c-Fos expression specifically in PKCδ+ CeL neurons (Figures S5G–S5J). These results suggest that DN glutamatergic neurons projecting to PKCδ+ neurons in the CeL play a bidirectional regulatory role in exercise-induced anxiety alleviation.
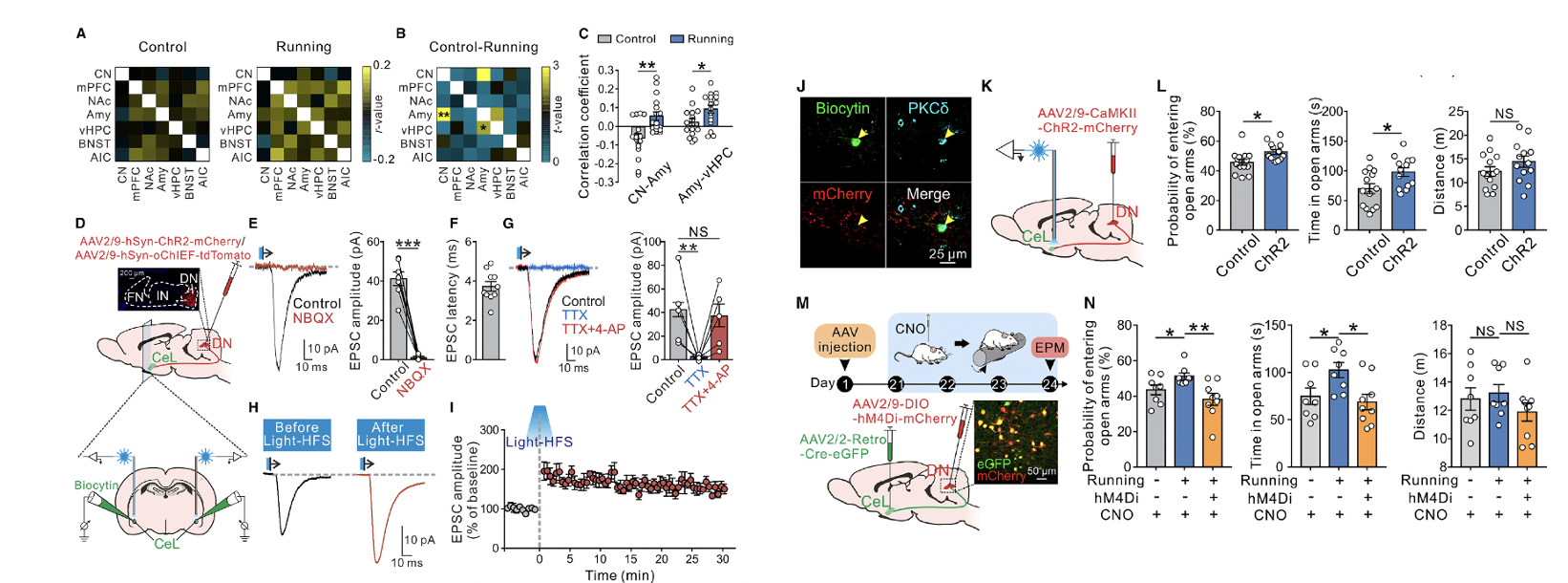
Figure 3. Monosynaptic projections from DN glutamatergic neurons to CeL PKCd+ neurons have long-term plasticity and mediate the improvement of anxiety by locomotion
Given the role of the neuropeptide orexin in exercise challenges and stress resilience, researchers further evaluated whether the hypothalamic orexinergic fibers known to project to the dentate nucleus (DN) contribute to the alleviation of CUMS-induced anxiety during running on a rotating rod. ELISA results showed that accelerated rotating rod running significantly increased orexin levels in the DN, whereas constant-speed running did not (Figure 4E). In contrast, PFA orexinergic neurons projecting to the CeL were not activated (Figure 4F), and the orexin release from PFA fibers in the CeL did not increase during exercise (Figure 4G). Moreover, the PFA orexinergic neurons projecting to the CeL were distinct from those projecting to the DN (Figures 4H–4J).

Figure 4. Challenging movements activate PFA-DN, but not PFA-CeL orexinergic projections
These findings suggest that direct PFA-CeL orexinergic projections are unlikely to contribute to the mechanism of exercise-induced anxiety relief. Using orexin-Cre rats, researchers demonstrated that optogenetic activation of PFA orexinergic terminals in the cerebellar DN significantly improved CUMS-induced anxiety (Figure 5D). Similarly, selective chemogenetic inhibition of PFA orexinergic inputs to the DN markedly attenuated the anxiolytic effect of high-intensity rotating rod running on CUMS-induced anxiety-like behavior (Figure 5F). Furthermore, pharmacological blockade (Figures 5G and 5H) or genetic knockdown (Figures 5I–5K) of the orexin receptor type 2 (OX2R), which mediates the excitatory effects of orexin on DN neurons, abolished the anxiolytic effect of high-intensity exercise.
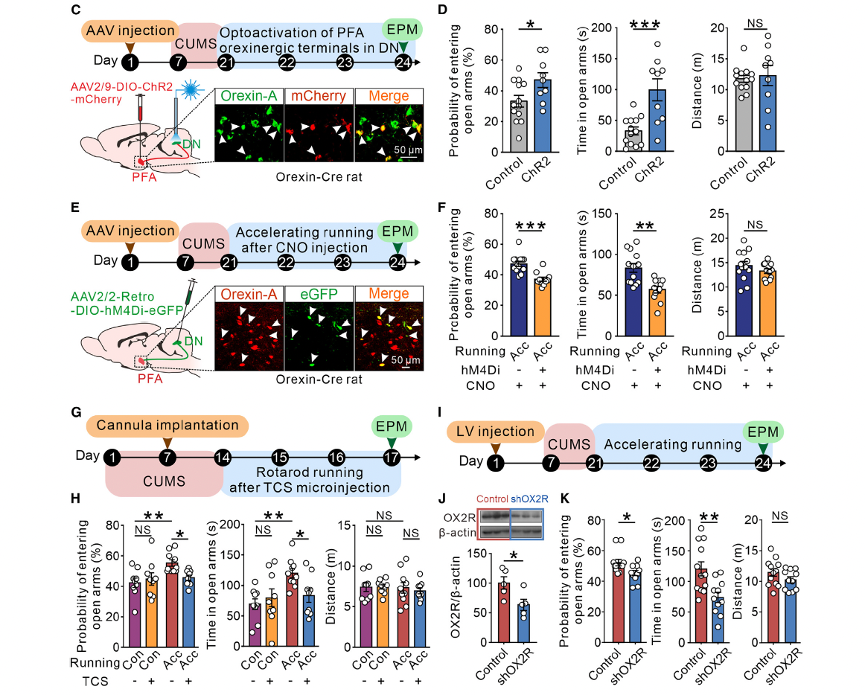
Figure 5. Challenging movements ameliorate stress-induced anxiety via activation of PFA-DN orexinergic projections
In summary, these findings indicate that the orexinergic hypothalamic-cerebellar pathway may play a crucial role in the alleviation of CUMS-induced anxiety by high-intensity exercise.
To investigate the synaptic effects of hypothalamic orexinergic fibers on cerebellar dentate nucleus (DN) neurons, researchers performed whole-cell patch-clamp recordings in cerebellar slices. Perfusion of orexin-A, a splice variant of orexin, excited glutamatergic projection neurons in the DN but had no effect on the activity of GABAergic neurons or interneurons in the DN (Figures 6A, 6B, and Figures S6A–S6E). During intense exercise challenges, PFA-DN (orexinergic neurons from the PFA projecting to the DN) were significantly activated (Figure 4D). Researchers further examined whether manipulation of orexinergic inputs impacts the spiking activity of DN neurons, particularly DN-CeL neurons, in a manner consistent with these findings.
As shown in Figure 6D, in orexin-Cre rats, Cre-dependent expression of eNpHR-mCherry and ChR2-eYFP was achieved in PFA orexinergic neurons and DN-CeL neurons, respectively. Using this method, they found that DN-CeL neurons expressing ChR2 showed increased spiking activity upon blue light activation (Figure 6D). Conversely, optogenetic inhibition of PFA-DN orexinergic terminals expressing eNpHR-mCherry using yellow light significantly suppressed the firing rate of DN-CeL neurons. These effects were particularly pronounced during accelerated rotating rod running and were not observed during constant-speed running (Figures 6E and 6F).
In vivo multichannel recordings demonstrated that intracerebellar injection of the selective orexin receptor type 2 (OX2R) antagonist TCS-OX2-29 effectively blocked hypothalamic orexinergic input, abolishing the increased firing rate of DN neurons during accelerated rotating rod running, while having no impact on their firing rate during constant-speed running (Figures S7A–S7C). Furthermore, broad chemogenetic inhibition of DN-CeL projections nearly completely abolished the anxiolytic effects of challenging exercise (Figures S7F–S7H). Specific chemogenetic inhibition of glutamatergic DN neurons, directly regulated by PFA orexinergic inputs and projecting to CeL PKCδ+ neurons, effectively blocked the anxiolytic effects induced by optogenetic activation of PFA-DN orexinergic terminals (Figures 6G and 6H).
In summary, challenging exercise effectively alleviates stress-induced anxiety by activating the orexin-driven hypothalamic-cerebellar-amygdalar (PFA-DN-CeL) circuit.

Figure 6. The orexin-mediated superimposed activation of DN-CeL circuitry contributes to the better anxiolytic effects of challenging exercise
Additionally, the article highlights that providing appropriate levels of exercise challenge can optimize the effectiveness of anxiety relief. It further explores whether the cerebellar nuclei could serve as a potential central target for brain stimulation to alleviate anxiety.
For those particularly interested in this article, the original text can be accessed at https://doi.org/10.1016/j.neuron.2024.01.007.
The viral vectors used in this study can be inquired about by leaving a message on our official website.