- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Parathyroid hormone (PTH) is one of the key hormones regulating bone metabolism and calcium balance. However, how the central nervous system regulates PTH remains unclear. The subfornical organ (SFO), located above the third ventricle, is known to regulate fluid balance. Using retrograde tracing, electrophysiological experiments, and in vivo calcium imaging, researchers identified the SFO as a critical brain nucleus in mice that responds to changes in serum PTH levels. Blocking different PTH receptors in the SFO was found to affect peripheral PTH levels and its response to calcium stimulation. Further studies revealed that GABAergic projections from the SFO to the paraventricular nucleus can regulate PTH levels and bone mass. These findings expand our understanding of the central nervous system’s regulatory mechanisms for PTH at the cellular and neural circuit levels.
Previous studies have shown that the PTG is innervated by sympathetic, parasympathetic, and sensory nerves; however, the central origins of these innervations remain unclear. To identify the brain nuclei that innervate the PTG, researchers directly injected the retrograde neural tracer pseudorabies virus (PRV-GFP) into the PTG (Figures 1A–1G). It was observed that PRV spread through the brainstem to infect the CNS, starting with the intermediate reticular nucleus (IRt) and the raphe magnus nucleus (RMg) (Figures 1C and 1D). By day 7 post-infection, brain nuclei with high PRV expression included the median preoptic nucleus (MnPO), the subfornical organ (SFO), the suprachiasmatic nucleus (SCh), the vertical limb of the diagonal band (VDB), and the arcuate nucleus (Arc) (Figures 1F and 1G; Figure S1).
The MnPO, SFO, and area postrema (AP) belong to a group of structures called circumventricular organs (CVOs), characterized by an extensive and highly permeable microvascular network lacking a blood-brain barrier.
To determine whether peripherally administered PTH can enter the CNS and directly bind to brain tissue, researchers performed a PTH-biotin binding assay. Immunofluorescence (IF) staining was used to detect biotin in the brain, identifying binding-positive regions. These regions included the SFO, periventricular thalamic nucleus (PV), SCh, periventricular hypothalamic nucleus (Pe), AP, and nucleus of the solitary tract (NTS) (Figures 1H–1J; Figure S2). Most identified nuclei were located near ventricles, indicating that peripheral PTH can cross into the CNS and bind to specific brain nuclei.
To assess neuronal activation, human PTH (1–34) or saline was intraperitoneally (i.p.) injected into C57 mice, and cFos immunostaining was used to evaluate activated neurons. Results showed that neurons in multiple brain nuclei, including the SFO, paraventricular nucleus (PVN), anterior paraventricular thalamic nucleus (PVA), and SCh, were activated in response to serum PTH (Figures 2A, 2C, and 2D; Figure S3). Notably, the number of cFos-positive cells significantly increased in the SFO and PVN (Figure 2B).
Using these three methods, the researchers confirmed that the SFO is a major brain nucleus both physically and functionally connected to the PTG.

Figure 1&2. Parathyroid glands are connected to the SFO and PVN(1). Parathyroid hormone activates SFO(2).
To investigate whether SFO neuronal activity regulates serum PTH levels, researchers directly activated SFO neurons and analyzed changes in serum PTH (Figure 3A). Using virus-mediated optogenetic and chemogenetic approaches, they selectively activated glutamatergic neurons and GABAergic neurons in the SFO. Activation of these neurons resulted in a reduction in serum PTH levels, indicating that PTH secretion directly responds to SFO neuronal activity.
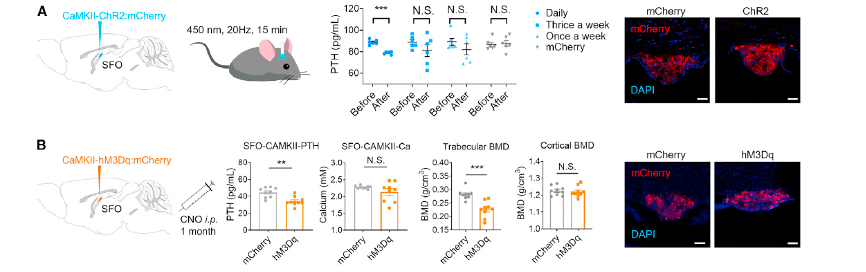
Figure 3. SFO neurons regulate serum PTH level
Chronic activation of these neurons significantly reduced serum PTH levels. This was accompanied by a decrease in bone mineral density (BMD) in the tibial trabecular bone compared to the control group, along with marked changes in the serum bone marker alkaline phosphatase (ALP), indicating an enhanced bone remodeling process.
These findings demonstrate that both SFO-GAD and SFO-VGlut neurons regulate serum PTH levels and bone metabolism. However, the roles of these two neuronal populations are antagonistic in PTH regulation and bone remodeling, suggesting that the CNS employs distinct pathways to bidirectionally regulate PTH levels.
Peripheral PTH can directly bind to and activate SFO neurons (Figure 1H). Researchers further evaluated the expression of PTH receptors in the SFO and their role in regulating serum PTH levels. Co-localization staining revealed that both PTH1R and PTH2R are expressed in SFOVGlut and SFOGAD neurons (Figures 4A–4C). Neurons expressing PTH2R are widely distributed throughout the central nervous system, including the locus coeruleus (LC), amygdala, and periaqueductal gray (PAG), where they are associated with pain and nociceptive behaviors.
Using PTH1R-Cas9 or PTH2R-Cas9, researchers knocked down the expression of PTH1R and PTH2R in the SFO (Figure 4D). In the PTH1R knockdown group, serum PTH levels were 20.4% lower than in the control group, whereas PTH levels in the PTH2R knockdown group were comparable to the control group (Figures 4D and 4E). Total serum calcium levels remained unaffected across all groups (Figure 4E).
Micro-CT analysis of the effects of PTH1R/PTH2R knockdown in the SFO on bone showed that the bone mineral density (BMD) of tibial trabecular bone and femoral cortical bone was not affected. However, in the PTH1R knockdown group, trabecular number (Tb.N) increased, and trabecular spacing (Tb.Sp) decreased compared to the control group (Figures 4F–4I).
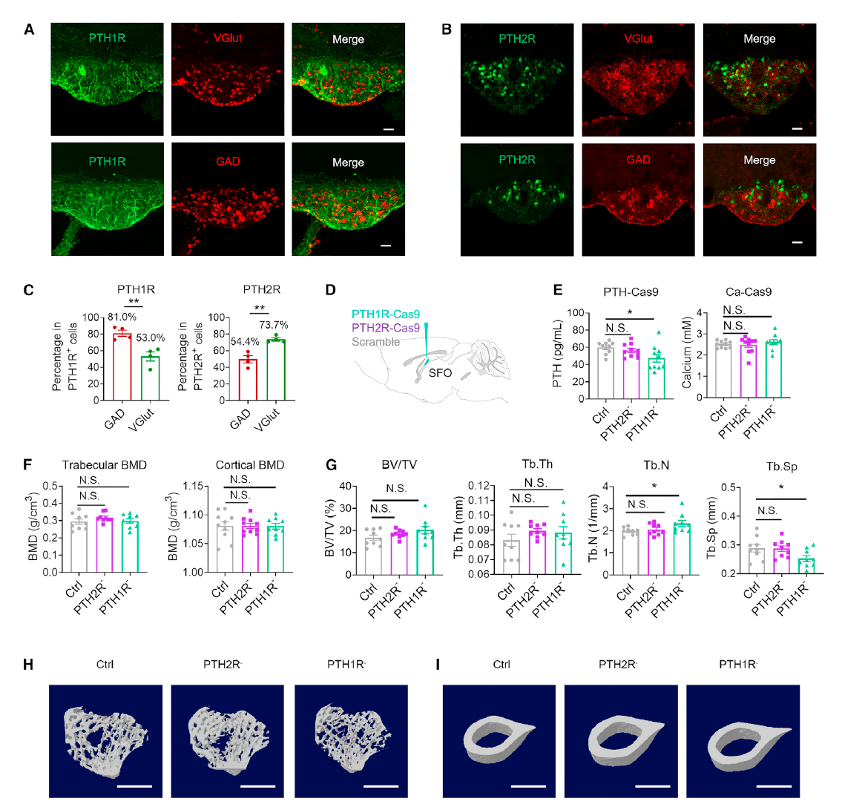
Figure 4. The function of PTH1R and PTH2R in the SFO
To assess the response to hypercalcemia, animals were intraperitoneally injected with 3 mM CaCl2. In the control group, acute elevation of calcium levels caused serum PTH levels to steadily decrease from A15 to A30, while serum calcium levels rose at A5, quickly dropped, and returned to normal by A30. However, in the SFOPTH1R and SFOPTH2R knockdown groups, PTH levels increased following calcium stimulation, peaking at A15 and then declining, indicating a delayed PTH regulatory response.
These results suggest that knockdown of PTH receptors in the SFO affects not only basal PTH secretion but also the kinetics of PTH response to hypercalcemia.
In summary, PTH1R and PTH2R play distinct roles in regulating basal PTH secretion, maintaining PTH homeostasis in response to exogenous CaCl2 stimulation, and bone remodeling.
The researchers next explored downstream effectors of SFO neurons, including the medial preoptic area (MnPO), paraventricular nucleus (PVN), organum vasculosum of the lamina terminalis (OVLT), and bed nucleus of the stria terminalis (BNST) (Figure S9). Among these, the PVN plays a critical role in regulating neuroendocrine homeostasis and sympathetic activity. Thus, the study focused on whether PVN neurons are involved in the regulation of PTH and bone metabolism.
Unlike the SFO, which contains both GABAergic and glutamatergic neurons, the PVN neurons responding to peripheral PTH stimulation (cFos-positive) were predominantly co-localized with VGlut2, rather than GAD1 or GAD2 (Figure 5A). To assess the functional role of PVN glutamatergic neurons, researchers used VGlut2-Cre mice and injected AAV9-DIO-hM3Dq-mCherry to chemogenetically activate VGlut neurons in the PVN (Figure 5D).
Sustained activation of PVN glutamatergic neurons resulted in a significant increase in serum PTH levels, 55.0% higher than in the control group, and a 35.4% increase in tibial trabecular bone mineral density (BMD) (Figures 5D and 5F). Additionally, the experimental group exhibited a significant increase in trabecular bone volume fraction (BV/TV) and a reduction in trabecular separation (Tb.Sp) (Figures S10F and S10G).
These findings demonstrate that activation of PVN glutamatergic neurons effectively upregulates PTH levels and promotes bone remodeling.
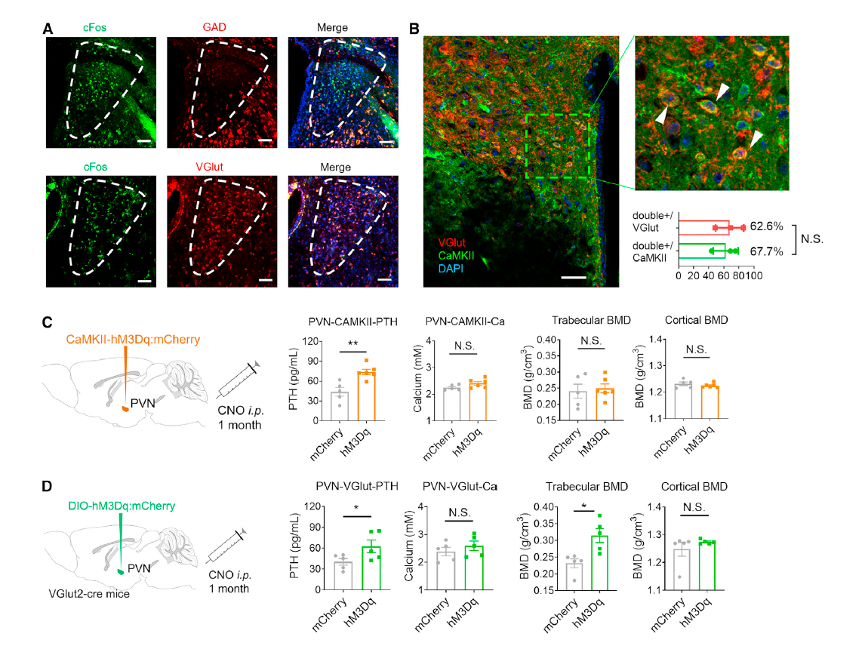
Figure 5. PVN neurons regulate serum PTH level
Zimmerman et al. previously observed direct projections from CaMKII neurons in the SFO to the PVN. However, it was unclear whether the PVN also receives projections from GABAergic neurons in the SFO. Through circuit tracing, the researchers demonstrated that the PVN receives dense projections from GABAergic neurons in the SFO (Figure 6A).
To verify whether glutamatergic neurons in the PVN form synaptic connections with GABAergic neurons in the SFO, they conducted retrograde tracing by injecting AAV9-DIO-TVA-RVG and RV-DG-mCherry into the PVN of VGlut2-Cre mice (Figure 6B). The presence of mCherry signals in the PVN confirmed successful transfection of PVN glutamatergic neurons by AAV helper and RV viruses. In the SFO, RV-positive mCherry signals co-localized with GABA and CaMKII markers, confirming a retrograde monosynaptic connection between PVN glutamatergic neurons and SFO GABAergic neurons (Figure 6B).
Dynamic interactions between the SFO and downstream brain nuclei were further explored by injecting DIO-ChrimsonR into the SFO of GAD2-Cre mice and recording calcium fluorescence signals in the PVN, LH, and OVLT (Figures 6C and 6D). Inhibitory signals in the PVN were more pronounced than those in the LH and OVLT, indicating that the neural circuit projecting from SFO GABAergic neurons to the PVN plays a critical role in PTH and bone mass regulation (Figure 6D).
Specific chemogenetic activation of the SFO GABAergic neuron projections to the PVN showed a 20.8% decrease in serum PTH levels and an 18.4% reduction in serum calcium levels (Figure 6E). Tibial trabecular bone mineral density (BMD) decreased by 5%, whereas femoral cortical BMD remained unaffected (Figure 6E).
These results reveal a specific SFO-GABA/PVN neural circuit that regulates serum PTH, total calcium, and trabecular bone metabolism.
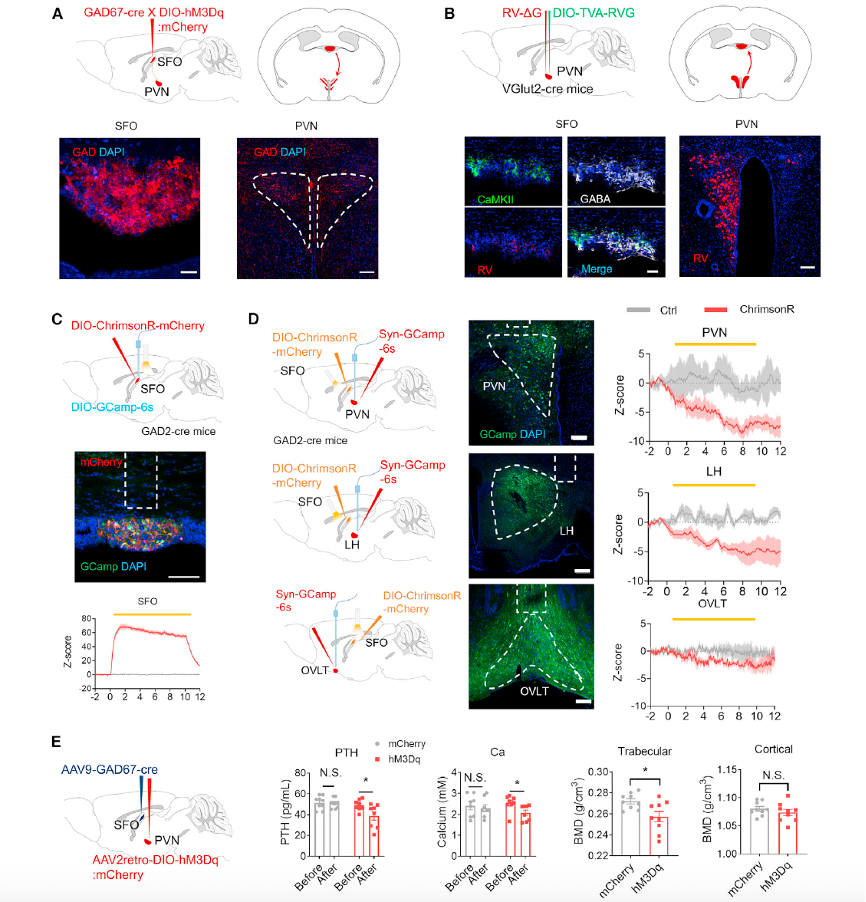
Figure 6. SFOGAD neurons send projections to the PVN
In this study, sympathetic nerves (TH+) and sensory nerves labeled with calcitonin gene-related peptide (CGRP+) were observed in mouse PTGs (Figures 7A and 7B). Double staining showed that sympathetic nerves formed a reticular structure surrounding blood vessels (CD31+) in the PTGs, clustering with vasculature rather than being uniformly distributed (Figure 7B). In contrast, most CGRP nerves appeared as single nerve fibers located between parathyroid structures (Figure 7B).
Tracing techniques revealed connections between the PTGs and the superior cervical ganglion (SCG) as well as the vagus nerve ganglia. Direct synaptic connections between the sympathetic and sensory nervous systems were suggested and further validated using a retrograde monosynaptic rabies tracer system.
To investigate the role of peripheral sympathetic nerves in PTH regulation, 6-hydroxydopamine (6-OHDa) was used to ablate peripheral sympathetic nerve terminals. Following systemic injection of 6-OHDa in mice, the percentage area of sympathetic nerve terminals in the PTGs decreased by 74.8%, and local ablation in rats reduced the percentage area by 71.6% (Figures 7I and 7K).
Global ablation of sympathetic nerve terminals in mice resulted in a decrease in baseline serum PTH levels (Figure 7L, left). Additionally, the serum PTH response to hypercalcemic stimulation was abolished (Figure 7L, left). In rats, local ablation of sympathetic nerve terminals in the PTGs did not affect baseline serum PTH levels; however, the 6-OHDa group showed no serum PTH response to hypercalcemic stimulation compared to controls (Figure 7L, right).
These findings consistently indicate that sympathetic and sensory nervous systems are closely integrated with the parathyroid glands. Peripheral sympathetic denervation significantly affects the PTH response to hypercalcemic stimulation.
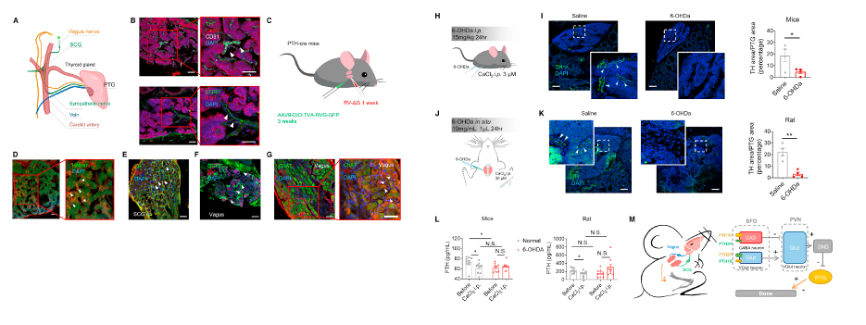
Figure 7. Peripheral innervation in parathyroid glands and sympathetic regulation of PTH
Click to fill in the requirements and submit them to us!