- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Social isolation stress is a widespread and pervasive issue in modern society. Even before the unprecedented COVID-19 pandemic, social isolation was prevalent globally, affecting nearly 40% of the population in some countries. During the COVID-19 pandemic, prolonged social isolation was found to have adverse effects on both physical and mental health, extending the impact of the pandemic beyond the virus itself.
Like other chronic stressors, social isolation can reshape the brain and trigger abnormal behaviors, including impaired social competence and anxiety. Studies have shown that, unlike acute isolation, which promotes social motivation and prosocial behavior, chronic social isolation stress (CSIS) induces social avoidance behaviors in both humans and rodents. These chronic antisocial behaviors are thought to further impair fundamental social integration, leading to additional isolation and forming a maladaptive cycle of social withdrawal.
Currently, the neural mechanisms underlying CSIS-induced social avoidance, particularly in adults, remain poorly understood. CSIS is known to induce widespread changes in the brain. A pioneering study using the expression of the stress-activated neuropeptide tachykinin 2 (Tac2)/neurokinin B (NkB) identified five brain regions with significant changes following CSIS in adulthood. Additionally, previous research has suggested that other brain regions might contribute to behavioral changes caused by both acute and chronic social isolation, including alterations in the dorsal raphe dopaminergic system and prefrontal cortex dysfunction. However, our understanding of the specific brain regions involved in CSIS-induced impairments in social competence remains limited.
Among these reported regions, studies suggest that neurotransmitter signaling in the anterior cingulate cortex (ACC) may regulate various social behaviors. Previous research (Guo et al.) demonstrated that dysfunctions in excitatory or inhibitory synapses in the ACC can cause social deficits in neurodevelopmental disorder models. Interestingly, the ACC is also one of the five brain regions showing the most significant changes. However, the role of synapses in the ACC in CSIS-related behavioral abnormalities, particularly social impairments, has yet to be clarified.
The cannabinoid type-1 receptor (CB1R) is highly expressed in prefrontal cortical associative areas and has been shown to regulate social competence. CB1R modulates synaptic transmission in several “social brain” regions, including the ACC, through activation by endogenous cannabinoids (endocannabinoids). Moreover, accumulating evidence suggests that pharmacological or genetic manipulation of CB1R affects social behavior in rodents. Additionally, studies using semi-quantitative analyses have shown altered endocannabinoid signaling in the brain following social isolation stress. However, it remains unclear whether CB1R-mediated synaptic and circuit remodeling plays a role in CSIS-induced social deficits.
In this study, researchers screened brain regions affected by CSIS and identified the ACC as a potential target contributing to social competence impairments. They found that CSIS enhances synaptic inhibition in the microcircuitry of pyramidal neurons in the ACC, particularly in inhibitory synapses originating from cholecystokinin (CCK)-expressing interneurons. Furthermore, the CSIS-induced increase in inhibition was driven by enhanced calcium influx into inhibitory axons, resulting from dysfunctional presynaptic CB1R activity in CCK interneurons. In group-housed mice, selective knockdown of CB1R expression in CCK interneurons in the ACC recapitulated CSIS-induced social avoidance behaviors. Conversely, restoring CB1R activity through optogenetic activation or administering a CB1R agonist rescued social competence and reduced social avoidance behaviors in CSIS-exposed mice.
CSIS-induced social impairments
The researchers employed a well-established two-week isolation protocol and assessed social interactions in group-housed (GH) mice and CSIS mice using two experimental methods (Figure 1A). In the three-chamber experiment, they found that CSIS mice spent less time in the chamber with a same-sex unfamiliar mouse compared to GH mice (Figure 1B). CSIS mice also showed lower preference scores for the social stimulus mouse and spent more time in the empty chamber than GH mice (Figure 1C). Additionally, the researchers found that two weeks of social isolation significantly impaired social behavior, and the effects of CSIS lasted for at least 28 days (Figure S1E, see supplementary materials). These data suggest that a two-week isolation can induce long-lasting social deficits in mice.
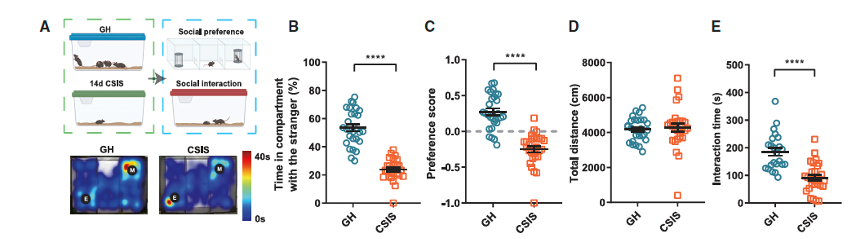
Figure 1. Chronic social isolation stress (CSIS) leads to social interaction impairments and synaptic inhibition enhancement in the ACC.
The researchers performed c-Fos staining on CSIS and GH mice after exposure to social stimuli (Figure S2B). They found a reduction in neuronal activation levels in the ACC (Figures S2C–S2G). Next, the researchers used optogenetic tools, specifically somBiPOLES, to bidirectionally regulate neural activity in five brain regions (Figures S2H and S2I). Interestingly, they found that social deficits were only reversed when the ACC was optogenetically activated (Figures S1J–S1S). These data suggest that the ACC may be a key target in studying the mechanisms underlying social dysfunction caused by CSIS. Previous research has shown that synaptic dysfunction in the ACC leads to social impairments associated with certain neurodevelopmental disorders.
Through patch-clamp recordings of pyramidal neurons in the ACC layer 2/3, the researchers found no significant changes in the frequency or amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) (Figures S3A–S3D), suggesting that excitatory synapses in the ACC were not affected by CSIS. However, the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) was significantly increased in CSIS mice, with no change in amplitude (Figures 1F–1H). During the first week of isolation, no significant increase in sIPSC frequency was observed, consistent with the timeline of behavioral changes induced by CSIS. Additionally, by blocking all synaptic inputs and recording the intrinsic properties of pyramidal neurons in the ACC, no significant changes were found (Figures S3E–S3N). These results suggest that CSIS may specifically affect the release of GABA in the ACC microcircuits without altering excitatory inputs or intrinsic neuronal properties.
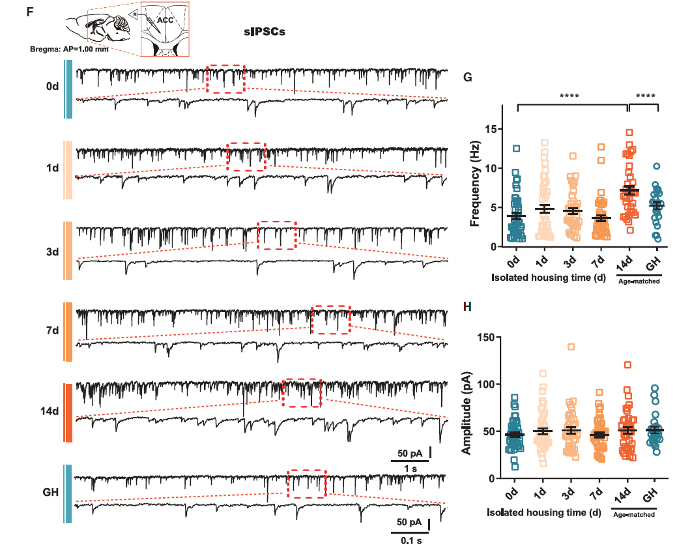
Figure 1. Chronic social isolation stress (CSIS) leads to social interaction impairments and synaptic inhibition enhancement in the ACC.
To explore the neural basis of the changes in GABA release, the researchers first examined whether the number of GABAergic neurons increased due to CSIS. Using GAD67-GFP transgenic mice to label GABAergic neurons, they found that the number of GFP+ neurons did not change (Figure S5).
Next, the researchers tested the function of GABAergic synapses. The results showed that, compared to PV-expressing interneurons and SST-expressing interneurons, stimulation of CCK-expressing interneurons in CSIS mice triggered a higher frequency of quantal events, while the amplitude remained unchanged (Figures 2A–2C). These data suggest that CCK-expressing interneurons are more vulnerable in CSIS, and the enhanced GABA release caused by CSIS is cell-type specific.
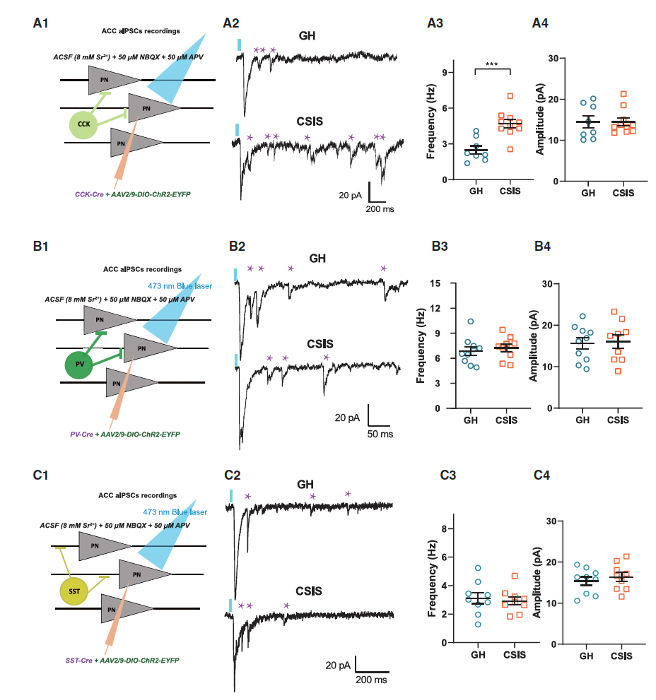
Figure 2. Abnormal inhibition is derived from CCK-expressing interneurons in CSIS mice
The researchers introduced stable gait-function optogenetic protein (SSFO) into CCK-expressing interneurons and used the mDlx enhancer to restrict Cre-dependent expression of SSFO (Figure 3A). Combined with c-Fos staining, they found that SSFO-eGFP+ neurons showed c-Fos signals in their nuclei, indicating neuronal activation (Figures 3B–3D). When blue light was applied, the frequency of sIPSCs in the SSFO expression group significantly increased, while the amplitude of sIPSCs remained unchanged (Figures 3F–3H). Additionally, compared to the control eGFP group, the number of c-Fos+ neurons was reduced in the SSFO expression group (Figure 3E). These results suggest that optogenetic activation of CCK-expressing interneurons increases GABA release in the ACC and inhibits local neuronal activity. Behaviorally, selective activation of CCK-expressing interneurons in the ACC could simulate social impairments similar to those caused by CSIS. Furthermore, optogenetic inhibition of CCK-expressing interneurons could restore social behavior in CSIS mice. These findings collectively establish a causal relationship between the functional role of CCK-expressing neurons and CSIS-induced social impairments.
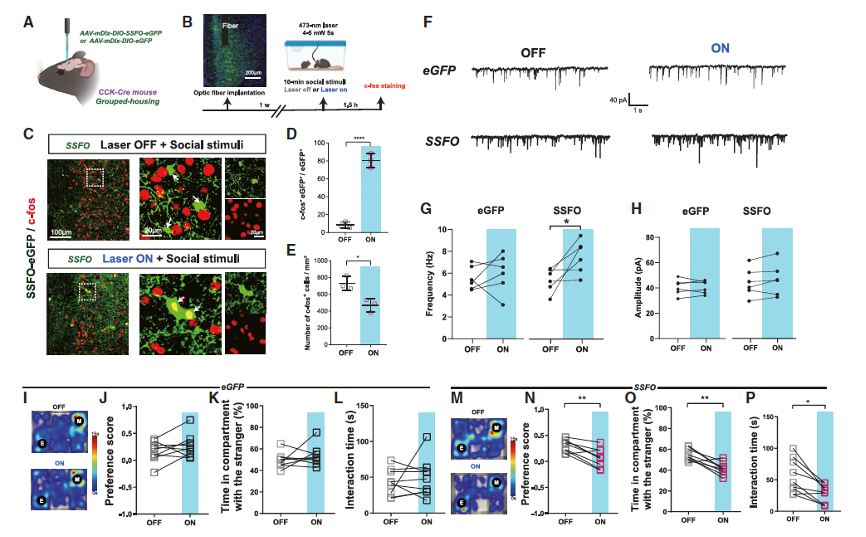
Figure 3. Optogenetic activation of ACC CCK-expressing interneurons suppresses social behaviors
The researchers next explored the mechanism underlying the vulnerability of CCK-expressing interneuron synapses after CSIS. Previous studies have shown that CCK-expressing interneurons form inhibitory GABA synapses near the soma with high expression of presynaptic CB1 receptors. When endogenous cannabinoids activate the presynaptic CB1 receptors, which are Gai/o-coupled protein receptors, the CB1 receptor reduces the probability of neurotransmitter release. By performing RNAscope in situ hybridization in GAD67-GFP transgenic mice, dual labeling Cck and Cnr1 mRNA, the researchers detected CB1 receptors specifically expressed in CCK-expressing interneurons. They found that Cck mRNA was expressed in both pyramidal neurons and GAD67-GFP-labeled interneurons. At the ultrastructural level, the researchers observed that CB1 receptors were distributed along the presynaptic membrane. Compared to GH mice, CSIS mice exhibited a significant decrease in CB1 receptor density at symmetric synapses, but no change was observed at asymmetric synapses (Figures 4D and 4E).
Next, to test the function of presynaptic CB1 receptors in inhibitory synapses, the researchers recorded depolarization-induced suppression of inhibition (DSI) mediated by presynaptic CB1 receptors and endogenous cannabinoids. After two weeks of social isolation, DSI suppression was significantly reduced compared to early isolation time points (Figure S10K), indicating impaired cannabinoid signaling after CSIS. This finding is consistent with the enhanced GABA release observed after 2 weeks of CSIS. To confirm the DSI changes in CCK-expressing inhibitory synapses, the researchers injected AAV carrying ChR2 into CCK-Cre mice. Importantly, compared to GH mice, the CSIS mice also showed impaired DSI (Figures 4F and 4G), which is consistent with the enhanced GABA release found in CCK interneuron synapses. These results suggest that the decreased expression of presynaptic CB1 receptors at inhibitory synapses leads to cannabinoid signaling dysfunction after CSIS.
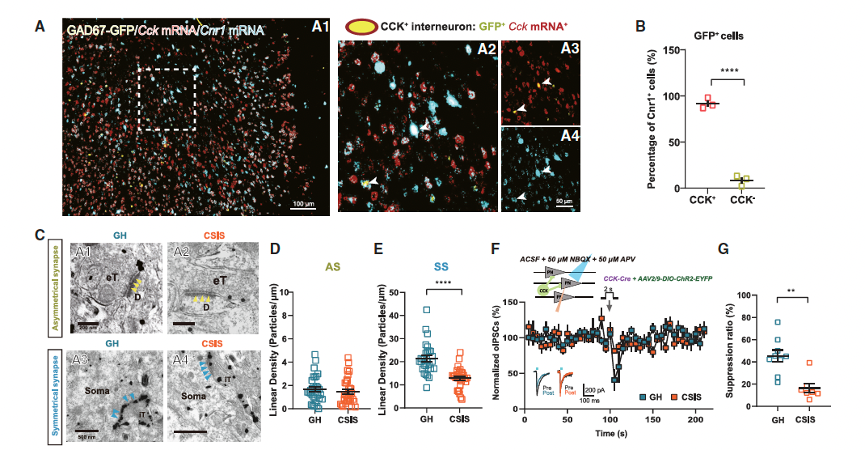
Figure 4. Reduced CB1Rs in CCK inhibitory synapses lead to increased GABA release via enhancing axonal calcium influx
Previous studies have reported that CB1R regulates GABA release by modulating calcium influx into GABAergic axon terminals. The researchers found that in CSIS mice, the frequency of calcium events was significantly higher than in GH mice during interactions with unfamiliar mice. After applying the CB1R agonist WIN 55,212-2, the calcium response amplitude in the CSIS group was comparable to that of the GH group. Additionally, WIN 55,212-2 was able to inhibit the frequency of sIPSCs in the CSIS mice. These results suggest that CB1R signaling dysfunction leads to increased axonal calcium influx in CCK-expressing interneurons after CSIS, resulting in enhanced GABA release.
The researchers isolated Cnr1 KD cells and performed patch-clamp recordings, finding that the DSI in the KD group was significantly reduced compared to the control group. In terms of social behavior, the KD mice spent significantly less time in the chamber with social stimulus mice, and their preference scores were notably lower than those of the control group. The combination of behavioral data and electrophysiological results indicates that conditional KD of CB1R in CCK-expressing interneurons is sufficient to trigger enhanced GABA release and social abnormalities similar to CSIS, suggesting a causal relationship between CB1R function in interneurons of the ACC and social behavior.
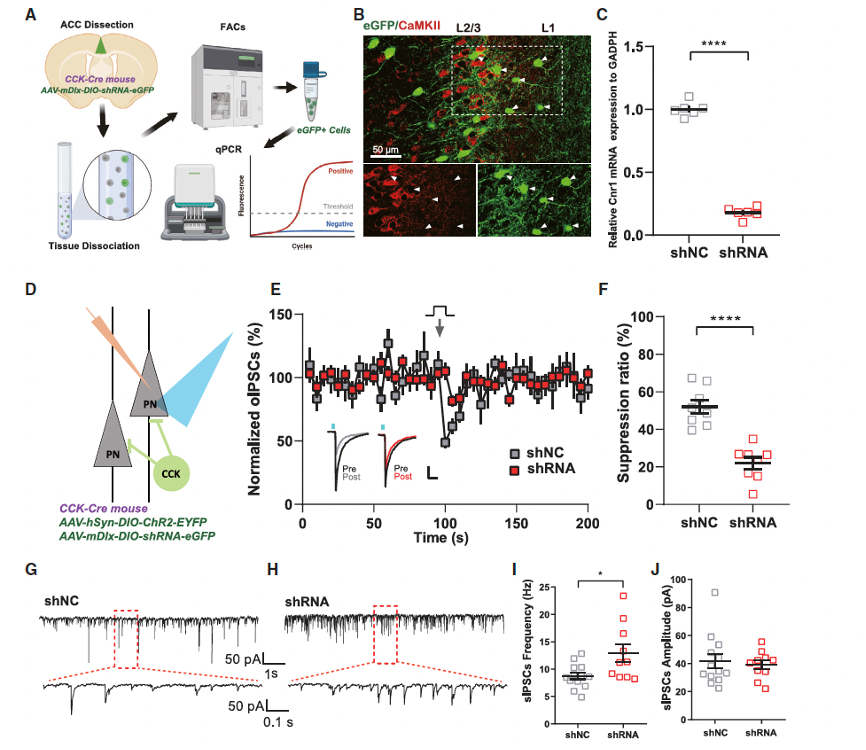
Figure 5. Conditional knockdown of CB1R in CCK-expressing interneurons mimics CSIS-like social impairments
Given that severe dysfunction of CB1R is a mechanism underlying the social deficits induced by CSIS, the researchers explored whether enhancing CB1R function could restore social abnormalities. To selectively activate CB1R in CCK-expressing interneurons, they used the opto-XR method, introducing opto-CB1R into CCK-expressing interneurons in the ACC of CSIS mice through a viral vector and implanted optical fibers to deliver blue light stimulation. Acute brain slice electrophysiological and behavioral experiments revealed that 20 Hz pulse stimulation successfully activated opto-CB1R, leading to a significant decrease in sIPSC frequency and a marked increase in social interaction time and preference scores. This enhanced social interaction time in CSIS mice when encountering unfamiliar juvenile mice.
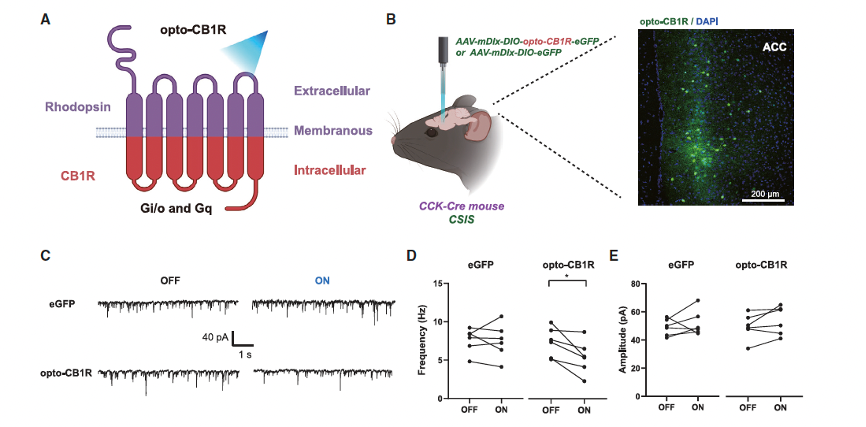
Figure 6. Photoactivation of CB1R in CCK-expressing interneurons restores social behaviors in CSIS mice
From a therapeutic perspective, the researchers also explored the effects of enhancing CB1R function with drugs. They found that the sIPSC frequency in the WIN 55,212-2 group was significantly lower than in the control group, with no significant difference compared to the GH control group. Regarding calcium signaling, they observed a reduction in the frequency of calcium events in CSIS mice after administering 1 mg/kg of WIN 55,212-2 using fiber photometry. To further confirm the involvement of CB1R in the ACC in the effects of systemic WIN 55,212-2 administration, they microinjected WIN 55,212-2 into the ACC and tested the social behavior of CSIS and GH mice. They found that local microinjection of WIN 55,212-2 could restore social dysfunction in CSIS mice, similar to the effects of systemic administration.
Click to fill in the requirements and submit them to us!