- E-mail:BD@ebraincase.com
- Tel:+8618971215294
As the first hormone to be discovered, secretin (SCT) was initially thought to be a humoral factor produced by the duodenum, responsible for pancreatic secretion and neutralizing pH in the duodenum. However, new data suggest that SCT, as a pleiotropic regulator, plays a role in energy homeostasis by controlling appetite, thermogenesis, and lipogenesis. In addition to its effects on energy metabolism, many clinical studies have also suggested a link between SCT and skeletal homeostasis. For example, one clinical study reported that serum SCT levels in postmenopausal women with type I osteoporosis were significantly lower than those in women with normal bone mass. Conversely, overexpression of the SCT gene helped reduce type I osteoporosis and increased bone mass in mice. However, supplementation of SCT to raise its circulating levels failed to promote new bone formation. These data suggest that SCT may not act directly on bone tissue but may instead influence bone homeostasis indirectly through the central nervous system (CNS), which is separated from the circulatory system by the blood-brain barrier.
In recent years, the presumed role of SCT as a neuropeptide in the CNS has attracted widespread attention, as it has been shown to be involved in regulating fluid homeostasis, motor function, and social behavior. Researchers recently used an SCT-Cre knock-in mouse model to confirm the abundance of SCT-expressing neurons in the ventromedial hypothalamus (VMH). It was demonstrated that SCT derived from VMH neurons maintains bone and energy homeostasis by targeting SCTR-expressing cells in the VMH. On one hand, systemic or conditional deletion of SCT derived from the VMH significantly increased sympathetic nervous activity (SNA), leading to a marked increase in bone loss in both male and female mice. On the other hand, SCT in the VMH also maintains energy balance by regulating appetite. VMH-specific deletion of SCT or SCTR leads to persistent hyperphagia, resulting in an obesity phenotype characterized by dysregulated lipogenesis and impaired thermogenesis. These findings reveal a previously unknown SCT signaling pathway in the VMH, which is crucial for the regulation of both energy metabolism and skeletal homeostasis.
To investigate the effects of SCT on energy metabolism, wild-type (WT) control mice, as well as whole-body knockout (KO) SCT (Sct−/−) and SCTR (Sctr−/−) mice, fed standard rodent chow, were raised to 20 weeks of age. It was found that the average daily food intake of freely fed Sct−/− and Sctr−/− mice was significantly higher than that of WT mice, particularly on the third day of the experiment (Figure 1a). Similarly, rebound food intake after fasting was also significantly higher in Sct−/− and Sctr−/− mice compared to WT mice (Figure 1b, c). However, the body weight of Sct−/− and Sctr−/− mice remained unchanged compared to WT mice over the entire 20-week period (Figure 1d).
Next, the researchers assessed the impact of whole-body SCT signaling on energy metabolism. When Sct−/−, Sctr−/−, and WT mice were fed a 60% high-fat diet (HFD), diet-induced obesity (DIO) was significantly observed in all three groups of mice (Supplementary Figure 3a). Consistent with previous observations, whole-body KO of SCT or SCTR resulted in hyperphagia, with cumulative food intake in Sct−/− and Sctr−/− mice being significantly higher than that in WT controls (Supplementary Figure 3b). Despite excessive hyperphagia, the body weight of Sct−/− and Sctr−/−mice was lower than that of WT mice starting from week 14 (Supplementary Figure 3a).
The researchers then conducted indirect calorimetry studies, and the results showed that during the dark cycle, carbon dioxide production (VCO2), oxygen consumption (VO2) (Figure 1f), and energy expenditure (EE) (Figure 1g) in Sct−/−and Sctr−/− mice were significantly higher than those in WT mice, while their locomotor activity was similar (Supplementary Figure 2e). These data suggest that compared to WT controls, sympathetic nervous activity (SNA) is upregulated in Sct−/− and Sctr−/− mice.
Given the important role of the sympathetic nervous system (SNS) in regulating bone homeostasis, the researchers then used micro-computed tomography (μCT) to measure bone mass in the distal femur bone marrow. The data indicated that the loss of SCT or SCTR resulted in significantly reduced trabecular bone volume fraction (BV/TV), bone mineral density (BMD), and trabecular thickness (Tb.Th), while specific bone surface (BS/BV) significantly increased (Figure 1i, j). In conclusion, SCT signaling maintains bone homeostasis by regulating sympathetic nervous activity.
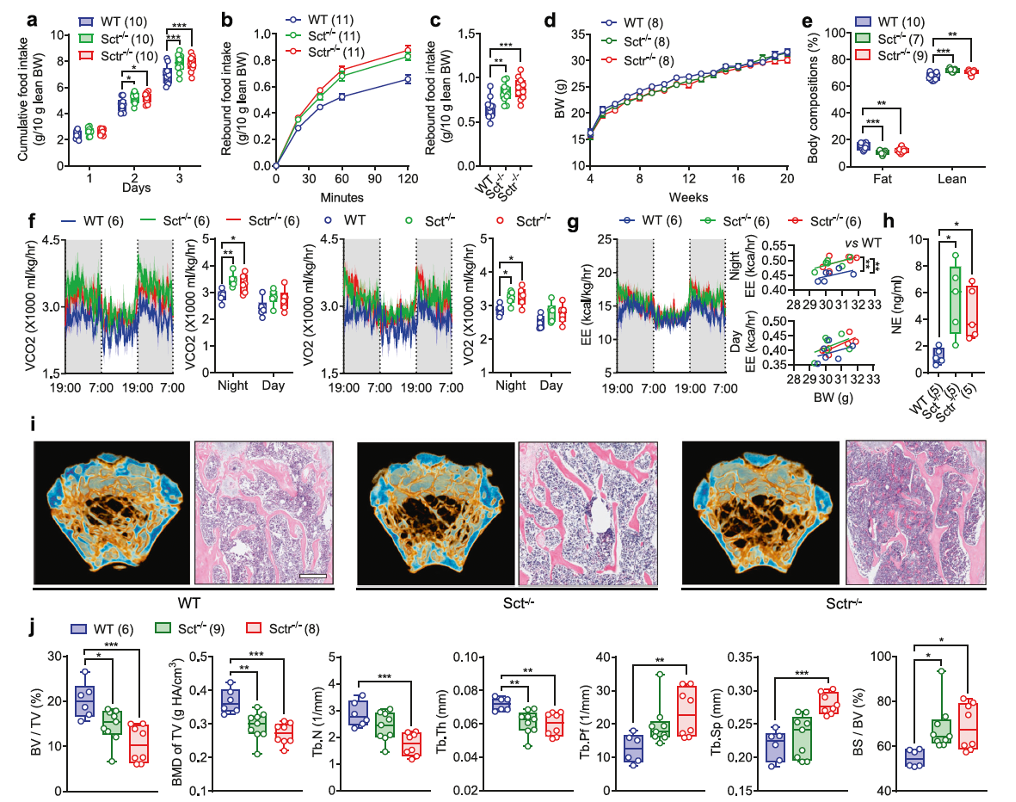
Fig. 1. Systemic SCT or SCTR KO results in metabolic dysfunction and bone loss
The ventromedial hypothalamus (VMH) plays a key role in both energy and bone homeostasis by regulating sympathetic nervous activity. By crossing SCT-IRES-Cre mice with R26-tdTomato mice, researchers found a dense distribution of SCT-expressing neurons in the VMH, and most Sct-positive signals were co-localized with steroidogenic factor 1 (SF-1; gene name Nr5a1), a major marker of VMH neurons.
To investigate the physiological role of SCT from the VMH, researchers employed a virus-mediated short hairpin RNA (AAV-ShSCT-eGFP, custom-made by Brain Case Biotech) approach to specifically inhibit the transcription of the Sct gene in the VMH. Similar to the whole-body SCT knockout, conditional knockdown of SCT in the VMH also led to a significant low bone mass phenotype, as evidenced by significantly reduced BV/TV, BMD of TV, Tb.N, and Tb.Th, along with significantly increased Tb.Pf, BS/BV, and Tb.Sp (Figures 2f, g).
Additionally, H&E staining not only confirmed the loss of trabecular structure but also showed excessive lipid accumulation in the bone marrow of ShSCT mice (Figures 2h, i). Tartrate-resistant acid phosphatase (TRAP) staining revealed a significantly higher number of osteoclasts in ShSCT mice compared to ShCon mice (Figure 2j). At the same time, the mineral apposition rate (MAR), labeled by fluorescent dyes, was significantly lower in ShSCT mice compared to ShCon mice (Figure 2k).
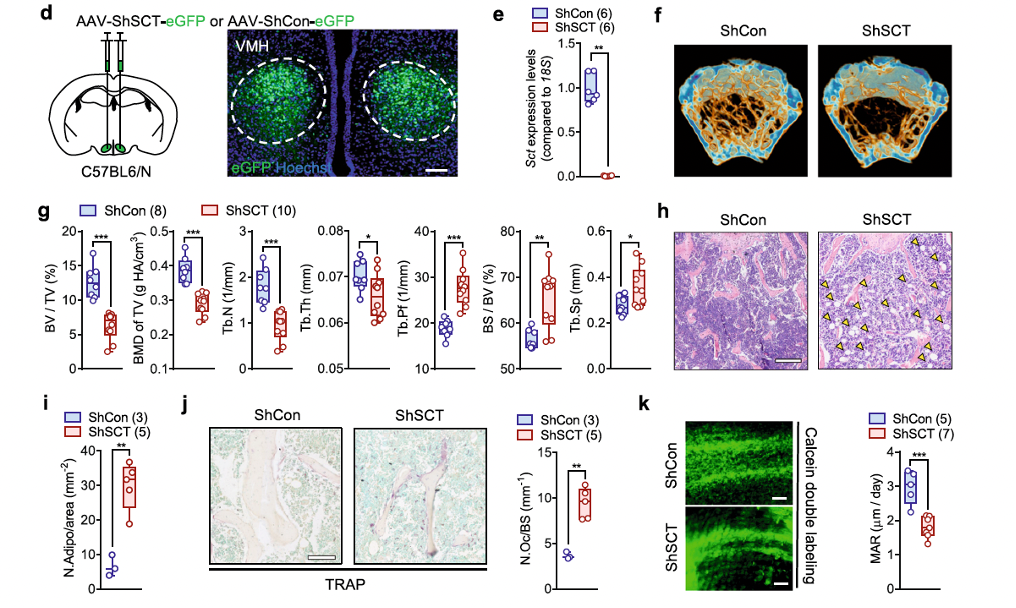
Fig. 2 | VMH-derived SCT regulate bone mass via SNS.
In conclusion, our results indicate that the osteoporosis observed in ShSCT mice is caused by increased osteoclastogenesis and reduced new bone formation, and SCT signaling in the VMH plays a critical role in maintaining bone mass.
In addition to its impact on bone homeostasis, researchers noticed that specific VMH SCT knockdown also caused significant disruptions in energy metabolism. Similar to the effects observed in Sct−/− and Sctr−/− mice, both male and female ShSCT mice exhibited increased daily food intake and rebound feeding (Figures 3a, b and Supplementary Figures 10a, b). Notably, although peripheral SCT administration suppressed appetite in fasted mice, it did not fully eliminate the hyperphagia observed in ShSCT mice (Figure 3c).
Subsequent analysis of appetite-related genes showed that the expression levels of appetite-stimulating genes, such as agouti-related peptide/neuropeptide Y (AgRP/NPY), in the hypothalamus of ShSCT mice remained unchanged, while the expression of the anorexigenic gene pro-opiomelanocortin (POMC) was significantly reduced (Figure 3d), suggesting that hyperphagia may be related to the downregulation of anorexigenic signaling in the hypothalamus.
Interestingly, despite the upregulation of sympathetic nervous activity, the body weight of ShSCT mice significantly increased (Figure 3e and Supplementary Figure 10c), with a higher fat mass ratio and lower lean body mass compared to ShCon mice (Figure 3f and Supplementary Figure 10d). Moreover, the loss of SCT from the VMH significantly increased the mass of inguinal white adipose tissue (iWAT) and epididymal white adipose tissue (eWAT), which are the primary fat storage sites in mice (Figure 3g). In the iWAT of ShSCT mice, researchers observed an increased number of hypertrophic adipocytes (Figure 3h), and the expression of thermogenic brown adipocyte marker genes was significantly downregulated, including uncoupling protein 1 (Ucp1), cytochrome c oxidase subunit 8b (COX8b), cell death activator CIDE-A (CIDEA), and fibroblast growth factor 21 (FGF21) (Figure 3i), indicating metabolic dysfunction and impaired browning of adipocytes.
Additionally, the serum insulin levels in ShSCT mice were significantly elevated compared to controls (Figure 3j). Associated with hyperinsulinemia, ShSCT mice displayed glucose intolerance and insulin resistance (Figures 3k–n). Consistent with these findings, SCT knockdown mediated specifically in the VMH (SCTVMH−/−) also resulted in hyperphagia (Figures 3o, p), significant weight gain (Figure 3q), and increased fat mass ratio compared to controls (Figure 3r).
In summary, SCT originating from the VMH plays a crucial role in the central perception of satiety and fullness, and the loss of SCT signaling leads to metabolic dysfunction and severe obesity.
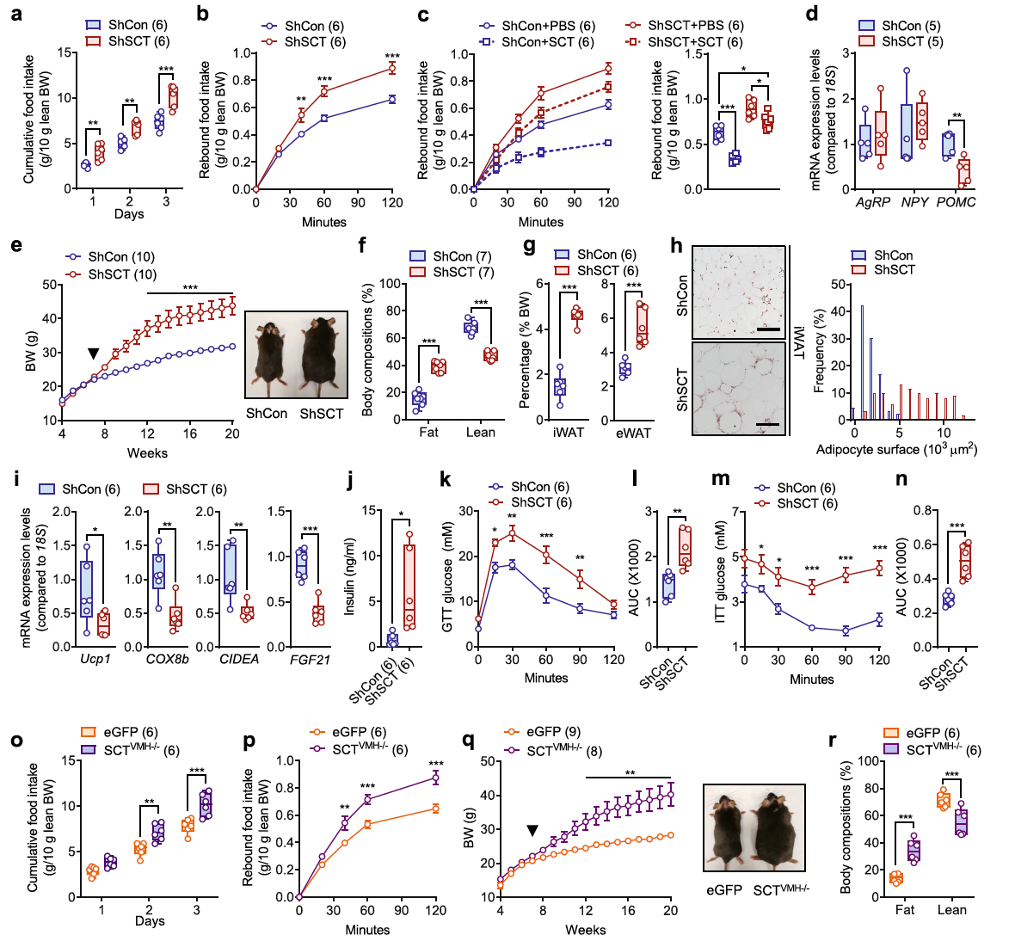
Fig. 3. Conditional KD of SCT in VMH leads to hyperphagia and obesity.
Energy homeostasis is achieved through strict regulation of both energy intake and energy expenditure by the central nervous system (CNS). The hypothalamus also maintains energy balance by regulating brown adipose tissue (BAT) thermogenesis via activation of the sympathetic nervous system (SNS). However, despite the systemic upregulation of sympathetic activity, researchers found that thermogenesis in the BAT of ShSCT and SCTVMH−/− mice was significantly impaired compared to control mice.
Specific VMH SCT knockdown resulted in a significant decrease in carbon dioxide output (VCO2), oxygen intake (VO2), and energy expenditure (EE) during both the dark and light cycles (Figures 4a, b and Supplementary Figures 6h, i). Histological observations showed increased fat accumulation in the interscapular brown adipose tissue (iBAT) of ShSCT mice, characterized by hypertrophic adipocytes (Figure 4d), increased tissue weight (Supplementary Figure 6k), and reduced protein density (Supplementary Figure 6l). Specific VMH SCT knockdown led to functional impairment of iBAT. Indeed, compared to ShCon mice, ShSCT mice exhibited lower mitochondrial content in iBAT (Figure 4e), as well as downregulation of genes related to thermogenesis or mitochondrial function. ELISA and Western blot analyses further confirmed reduced Ucp1 protein levels in the iBAT of ShSCT mice (Figure 4g and Supplementary Figure 6m). This finding is consistent with previous studies showing that genetic and/or pharmacological interventions in the hypothalamus can lead to impaired iBAT thermogenesis, accompanied by increased fat mass.
Furthermore, we found that the loss of SCT from the VMH led to chronic inflammation in iBAT, as indicated by the upregulation of macrophage marker genes (F4/80) and monocyte marker genes (MCP-1 and CD11c) (Figure 4h). Collectively, these data suggest that the loss of SCT in the VMH triggers whitening and inflammation of BAT, causing dysregulation of sympathetic innervation and weakening BAT’s response to SNS regulation.
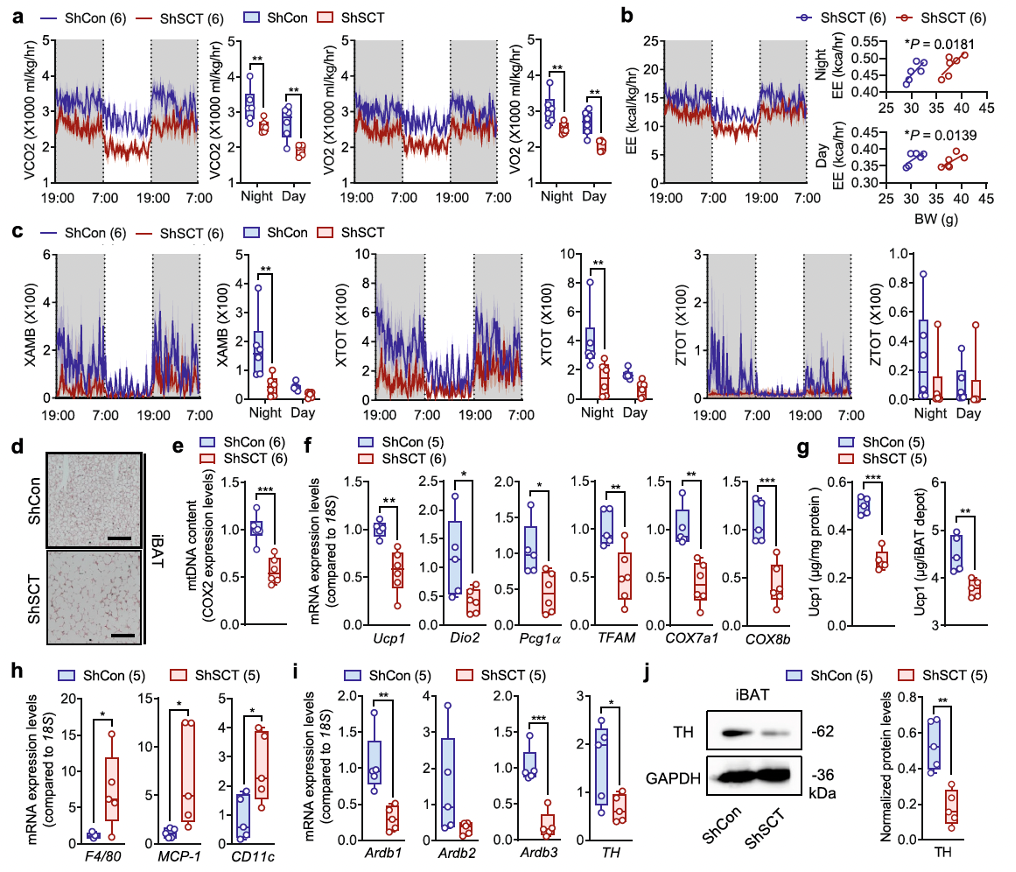
Fig. 4. Conditional KD of SCT in VMH leads to thermogenic dysfunction.
Obesity is closely linked to bone homeostasis through various signaling pathways involved in metabolic disorders. Thus, researchers sought to investigate whether VMH-specific SCT knockdown further exacerbates metabolic disorders and bone loss induced by a high-fat diet (DIO). After being fed a high-fat diet, both SCTVMH−/− mice and control littermates showed rapid weight gain. However, starting from the third week of high-fat diet feeding, the body weight of SCTVMH−/− mice exceeded that of eGFP mice (Figure 6a). Nevertheless, there were no significant differences in body composition (Figure 6b) or serum leptin levels (Figure 6c) between SCTVMH−/− mice and their eGFP littermates.
Similar to mice fed a standard diet, SCTVMH−/− mice fed a high-fat diet also exhibited significantly increased daily food intake (Figure 6d) and higher sympathetic nervous tension (Figure 6e). Additionally, indirect calorimetry revealed that compared to eGFP mice, SCTVMH−/− mice had significantly reduced VCO2, VO2, and energy expenditure (EE) (Figures 6f, g), as well as noticeably lower nighttime activity levels (Figure 6h). Furthermore, under the high-fat diet challenge, Cre-mediated SCT knockdown in the VMH led to further exacerbated bone loss and more severe lipid infiltration compared to control mice (Figures 6i–k). These findings suggest that in cases of obesity, the loss of SCT signaling in the VMH may be a key issue, as it exacerbates both energy and bone metabolism dysfunctions.
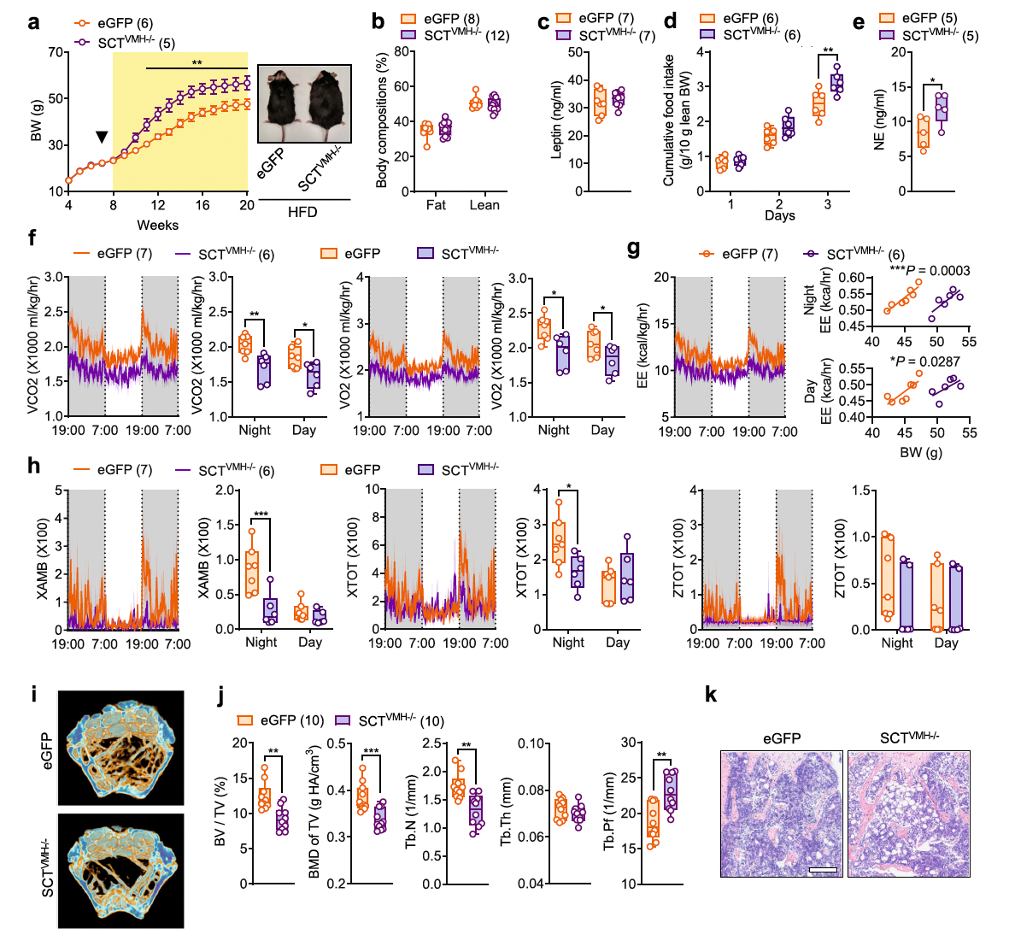
Fig. 6. Conditional KD of SCT in the VMH exacerbates obesity and osteopenia in DIO mice.
Since SCT deficiency in the VMH has been shown to lead to bone loss, the researchers investigated whether supplementation of SCT peptide in the VMH could promote bone mass increase. Through AAV-mediated overexpression of SCT (SCToe), they found that following the increase in SCT levels in the VMH (Figure 7b), the pCREB levels in the VMH also increased in SCToe mice (Figure 7c). Correspondingly, sympathetic nervous tension was significantly reduced in SCToe mice, as indicated by a notable decrease in serum norepinephrine (NE) levels compared to the mCherry control group (Figure 7d).
As a result, bone density at the distal metaphysis of the femur, measured by μCT, was significantly higher in SCToe mice than in control mice (Figures 7e, f). The increase in trabecular bone structure was further confirmed in histological studies (Figure 7g). Complementing this, the number of TRAP-positive cells in SCToe mice was significantly lower than that in mCherry mice (Figure 7h), suggesting reduced bone resorption activity.
In addition to the effects on bone homeostasis, the overexpression of SCT specifically in the VMH appeared to have little impact on energy balance. After the increase in SCT in the VMH, appetite (Figures 7i, j), body weight (Figure 7k), and body composition (Figure 7l) remained unchanged. This indicates that unlike the SCT-driven central control of bone homeostasis, which is closely mediated by the sympathetic nervous system, the VMH's perception of satiety can be maintained with minimal SCT. Thus, additional SCT beyond this level contributes little to appetite and energy metabolism regulation.
In summary, these results suggest that overexpression of SCT in the VMH reduces bone resorption and promotes increased bone mass in the femur of mice.
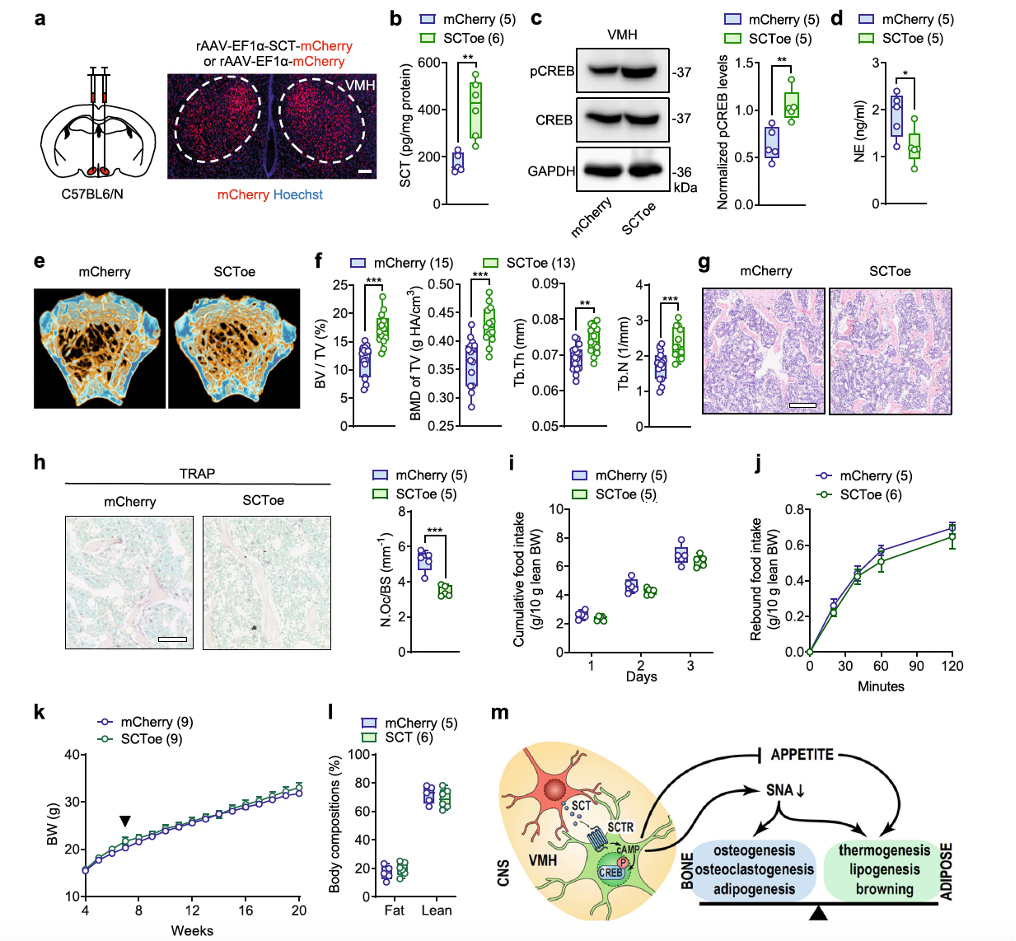
Fig. 7. SCT overexpression in VMH leads to bone mass increment.