- E-mail:BD@ebraincase.com
- Tel:+8618971215294
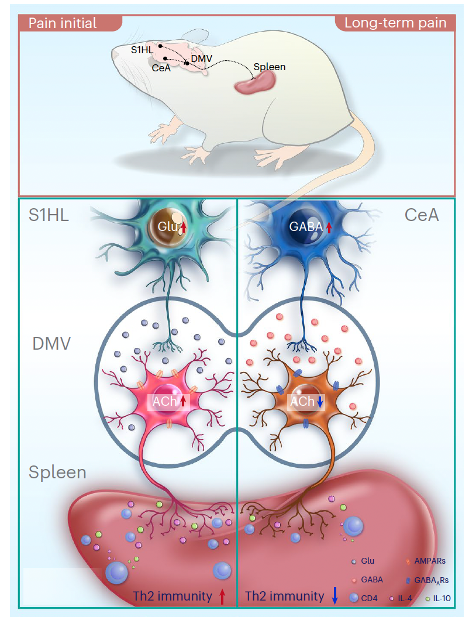
Pain involves interactions between the nervous and immune systems, but the mechanisms remain unclear. In this study, researchers demonstrate that the type 2 helper T cell (TH2) immune response in the spleen is regulated differently in male mice during acute and chronic neuropathic pain. By combining in vivo recordings and immune cell analysis, they reveal two distinct neural circuits related to pain-mediated peripheral TH2 immune responses: the glutamatergic neurons in the primary somatosensory cortex (GluS1HL) → AChDMV → spleen circuit, and the GABAergic neurons in the central amygdala (GABACeA) → AChDMV → spleen circuit. In the acute pain state, excitation from GluS1HL neurons to AChDMV neurons, which innervate the spleen, increases, leading to a higher proportion of TH2 immune cells in the spleen. In contrast, during chronic pain, inhibition from GABACeA neurons to AChDMV neurons increases, resulting in a reduction in TH2 immune cells in the spleen. Thus, our study reveals how the brain encodes immune responses in the spleen under specific pain conditions.
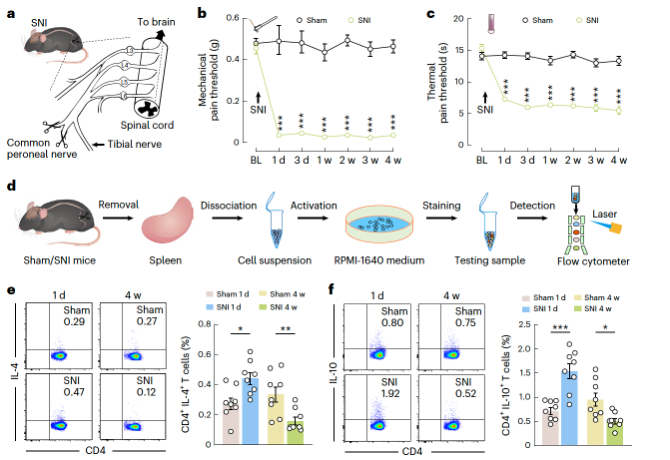
Researchers induced the classic spared nerve injury (SNI) model in mice and performed flow cytometric analysis on immune cells collected from the spleens of SNI model mice at 1 day, 1 week, 2 weeks, 3 weeks, and 4 weeks (as well as time-matched sham surgery controls). Compared to the corresponding sham controls, the proportion of CD4+ T cells increased in "acute pain" (SNI 1 day) mice, while the proportion of CD8+ T cells remained unchanged. In contrast, in "chronic pain" (SNI 4 weeks) mice, the proportion of CD4+ T cells decreased. It has been reported that TH1, TH2, and TH17 cytokines of CD4+ T cells are involved in pain processing. Flow cytometry revealed a significant increase in the proportion of TH2 cells expressing interleukin (IL)-4 and IL-10 in SNI 1-day mice compared to sham controls, while in SNI 4-week mice, these cells significantly decreased (Figure 1e,f). Morphine slow-release pellets were implanted at two time points—1 day and 3 weeks post-SNI surgery—and changes in the proportion of TH2 immune cells were observed following morphine treatment, indicating that pain sensitization induced by SNI affects TH2 immune cell alterations. This suggests that SNI-induced pain plays a role in regulating the number of TH2 immune cells in the spleens of SNI mice.
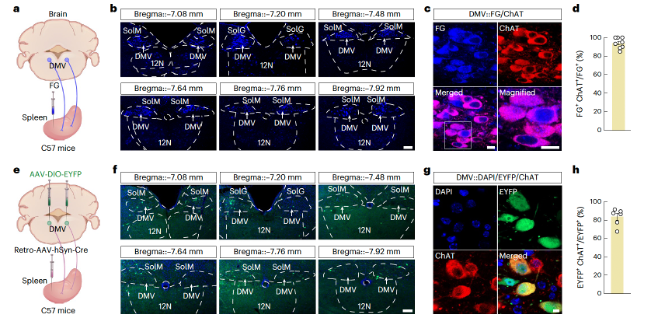
To explore the brain origins of the splenic nerves, we performed retrograde monosynaptic tracing by injecting Fluoro-Gold (FG) into the spleen (Figure 2a and Extended Data Figure 3a). One week later, FG+ signals were observed in the splenic nerve plexus and vagal efferent fibers labeled with peripheral nerve fiber markers (Extended Data Figure 3b,c. Extended Data can be found in the supplementary materials at https://doi.org/10.1038/s41593-023-01561-8). FG+ neurons were detected in the celiac ganglion (Extended Data Figure 3d) and the dorsal motor nucleus of the vagus (DMV, Figure 2b) and were co-labeled with choline acetyltransferase (ChAT) antibodies (Figure 2c,d). Notably, the DMV was previously shown to indirectly innervate the spleen through the DMV → celiac ganglion → spleen pathway. Therefore, they investigated the proportion of DMV neurons that project both directly and indirectly to the spleen. They performed retrograde monosynaptic tracing by injecting RV-N2C-ΔG-EGFP into the sympathetic celiac ganglion and FG into the spleen. One week later, they detected both EGFP+ neurons and FG+ neurons in the DMV (Supplementary Figure 2a,b). They found that approximately 43% of FG+ neurons also expressed EGFP signals (Supplementary Figure 2c), and about 83% of EGFP+ neurons were co-labeled with FG signals (Supplementary Figure 2d). These data suggest that DMV neurons innervate both the sympathetic celiac ganglion and the spleen, a result confirmed by altering the labeling strategy with FG injected into the celiac ganglion and RV-N2C-ΔG-EGFP injected into the spleen (Supplementary Figure 2e–h).
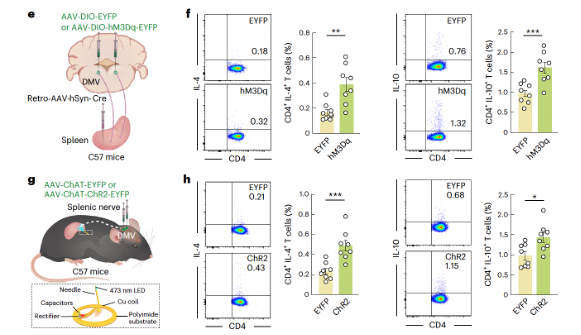
Researchers further investigated whether AChDMV neurons are involved in the spleen's TH2 immune response, previously observed in mice with neuropathic pain. One day after SNI, calcium signal recordings and chemogenetics were used to selectively monitor and regulate the activity of spleen-projecting AChDMV neurons. Researchers injected retrograde AAV-hSyn-Cre virus into the spleen and AAV expressing Cre-dependent fluorescent calcium indicator GCaMP6f (AAV-DIO-GCaMP6f) into the ipsilateral DMV, while installing a microendoscope GRIN lens above the DMV (Figures 3d, e and Supplementary Figures 6d–f). The results showed a significant increase in the frequency of calcium transients in these DMV neurons in SNI 1-day mice. Next, retrograde AAV-hSyn-Cre virus was injected into the spleen, and Cre-dependent excitatory hM4Di virus (AAV-DIO-hM4Di-EYFP) was injected into the DMV (Figures 3g, h and Supplementary Figures 6g–i). Three weeks after virus injection, CNO (which selectively inhibits spleen-projecting AChDMV neurons) was administered via intraperitoneal injection for seven consecutive days. The proportion of TH2 immune cells in SNI 1-day mice was significantly reduced compared to control mice. These results collectively indicate that the activity of spleen-projecting AChDMV neurons is enhanced upon induction of the SNI pain model.
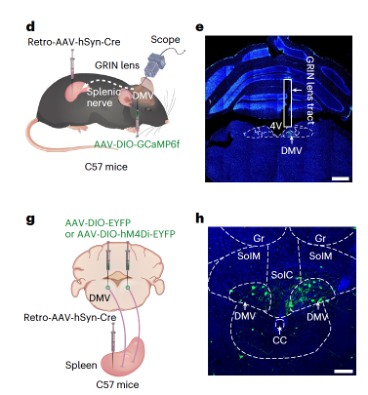
At 4 weeks post-SNI, researchers injected retrograde AAV-hSyn-Cre virus into the spleen and Cre-dependent excitatory hM3Dq virus (AAV-DIO-hM3Dq-EYFP) into the DMV. By selectively activating spleen-projecting AChDMV neurons with CNO through intraperitoneal injection, the results showed a significant increase in the proportion of TH2 immune cells in SNI 4-week mice compared to controls (Figures 4e, f and Supplementary Figures 7d–f). These findings suggest that although AChDMV neuron activity increases during early pain, its activity decreases in chronic pain. To rule out potential indirect effects on other visceral organs (such as the stomach) following chemogenetic activation of DMV neurons, wireless optogenetic experiments were conducted to specifically manipulate cholinergic terminals in the spleen (Figure 4g). It was found that wireless optogenetic activation of cholinergic terminals (Supplementary Figures 7g–i) significantly increased the proportions of CD4+ IL-4+ and CD4+ IL-10+ TH2 cells in SNI 4-week mice after activation of the AChDMV → spleen circuit. These results collectively confirm that the AChDMV → spleen circuit is involved in regulating the proportion of TH2 cells in the spleens of SNI 4-week mice.
Researchers examined how neuropathic pain affects the activity of the GluS1HL→AChDMV circuit and detected notable differences between SNI 1-day and SNI 4-week mice. Specifically, brain slice immunohistochemistry showed increased c-Fos expression in GluS1HL neurons projecting to the DMV (labeled by retrograde AAV-hSyn-EYFP injected into the DMV) in SNI 1-day mice, whereas no such expression was observed in SNI 4-week mice (Supplementary Figure 9). Additionally, whole-cell recordings in brain slices revealed enhanced activity of GluS1HL neurons projecting to the DMV in SNI 1-day mice compared to sham mice, but no such change was detected in SNI 4-week mice (Extended Data Figure 9).
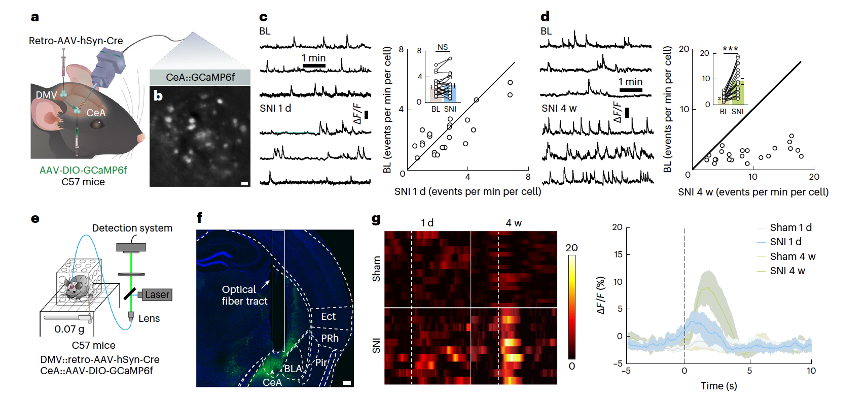
In tests of how neuropathic pain affects the GABACeA→AChDMV circuit, immunohistochemistry (Supplementary Figure 11) and whole-cell recordings in brain slices (Extended Data Figures 10a–f) demonstrated increased c-Fos expression and spike frequency in GABACeA neurons projecting to the DMV (labeled by retrograde AAV-hSyn-EYFP injected into the DMV) in SNI 4-week mice, but no differences were observed in SNI 1-day mice. These findings were confirmed through microendoscope imaging (Figures 5a–d, Supplementary Video 7) and calcium photometry recordings (Figures 5e–g), using retrograde AAV-hSyn-Cre injected into the DMV and AAV-DIO-GCaMP6f injected into the CeA
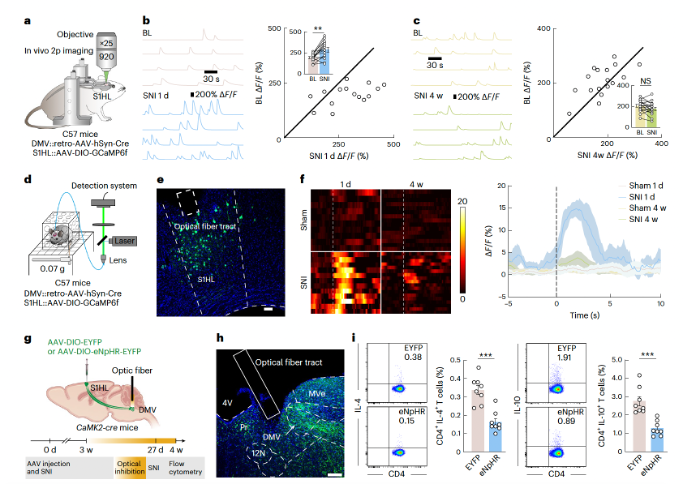
To visualize calcium signals in freely moving mice, researchers injected retrograde AAV-hSyn-Cre into the DMV and AAV-DIO-GCaMP6f into the S1HL (Supplementary Figures 10a–c). In vivo two-photon calcium imaging revealed higher fluorescence intensity in GluS1HL neurons in SNI 1-day mice compared to sham mice (Figures 6a, b), whereas no differences were observed in SNI 4-week mice (Figure 6c, Supplementary Video 4). To further explore whether GluS1HL neurons are sensitive to subthreshold pain stimuli, fiber photometry recordings were conducted in C57 mice following a similar tracing strategy (Figures 6d, e). After a 0.07g von Frey filament stimulation of the injured paw in SNI 1-day mice, there was a rapid increase in Ca2+ signals, while no such increase was observed in SNI 4-week mice (Figure 6f, Supplementary Videos 5 and 6).
Following the observed increase in GluS1HL→AChDMV→spleen circuit activity in SNI 1-day mice, researchers investigated whether artificial inhibition of this circuit would affect the TH2 immune response. By injecting Cre-dependent AAV carrying eNpHR (AAV-DIO-eNpHR-EYFP) into the S1HL of CaMK2-cre mice and implanting an optical fiber in the DMV to optically inhibit GluS1HL terminals, they found a significant reduction in the proportion of TH2 immune cells in the spleen (Figures 6g–i and Supplementary Figures 10d–f).
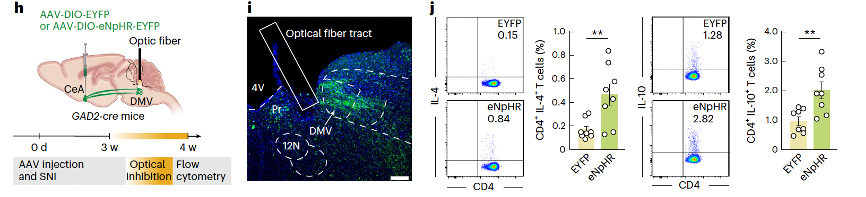
The increased activity of the GABACeA→AChDMV→spleen circuit in SNI 4-week mice led to an evaluation of whether artificial inhibition of this circuit would affect the TH2 immune response. In GAD2-Cre mice, AAV-DIO-eNpHR-EYFP was injected into the CeA, and optical inhibition of GABACeA terminals in the DMV was performed. This resulted in a significant increase in the proportion of CD4+ IL-4+ and CD4+ IL-10+ TH2 cells in the spleen of SNI 4-week mice (Figures 7h–j).
In summary, different neural circuits are involved in the immune response of the spleen in SNI 1-day mice (GluS1HL→AChDMV→spleen circuit) and SNI 4-week mice (GABACeA→AChDMV→spleen circuit) (Figure 8).