- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Major depressive disorder (MDD) is a significant public health issue. Many individuals diagnosed with MDD typically exhibit a persistent tendency toward anxiety and poor resilience under stress. Traditional antidepressant therapies, such as selective serotonin reuptake inhibitors (SSRIs), ketamine, and electroconvulsive therapy, have serious limitations. Scientists need to find more stable and fast-acting antidepressant targets and compounds. In recent years, experimental animal models of depression have shown that peripheral delivery of mesenchymal stromal cells (MSCs) can effectively alleviate depressive and anxiety-like behaviors. What are the potential mechanisms behind this effect?
Experiments have shown that most intravenously injected MSCs are distributed in the lungs. It is hypothesized that MSCs may interact with the numerous sensory neurons innervating the lungs and further transmit signals to the central nervous system. Researchers have found that peripheral delivery of MSCs activates pulmonary-innervating vagal sensory neurons, which project to the nucleus tractus solitarius, further inducing the release of 5-hydroxytryptamine (5-HT) in the dorsal raphe nucleus (DRN). Additionally, MSCs partially activate pulmonary-innervating vagal sensory neurons through the BDNF-TrkB signaling pathway. Inhalation of a TrkB agonist improved the abnormal behavioral patterns of CRS mice.
In the chronic restraint stress (CRS) model mice, compared to the CRS group, injection of bone marrow-derived MSCs significantly alleviated the weight loss associated with depression in this model (Fig. 1b). In the open field test (OFT), CRS mice showed little interest in entering the center area, while mice injected with MSCs exhibited significant increases in both the time spent and the distance traveled in the center area (Fig. 1c, d). Similar to the OFT results, CRS mice spent less time in the open arms of the elevated plus maze (EPM) compared to the control group, whereas MSC treatment (but not HDF injection) significantly increased the time spent in the open arms (Fig. 1e, f). The results from the OFT and EPM suggest that MSCs may have anxiolytic effects in CRS mice. Additionally, in the repeated social defeat (RSD) mouse model, the ability of MSCs to regulate social avoidance and anxiety-like behaviors in mice was detected (see the original supplementary materials).
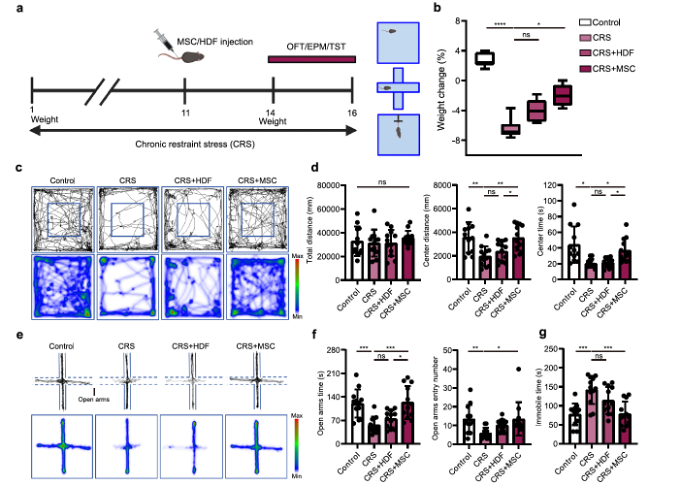
Fig. 1-1 MSCs attenuate depressive and anxiety-like behaviors.
Early studies have reported that MSCs improve aberrant behavioral patterns in mouse models of depression by modulating inflammatory processes. In this article, the researchers also examined whether the alleviation of depressive and anxiety-like behaviors in stressed mice treated with MSCs was due to anti-inflammatory effects. Their data indicated that the inflammation level in mice depends on the duration of stress, with MSCs showing significant anti-inflammatory effects only during the early stages of stress. However, after 14 days of CRS or 7 days of RSD, IL-6 and TNF-α levels in the stress-treated mice were not significantly different from those in the normal and cell therapy groups. Stress-induced depressive and anxiety-like behaviors are commonly associated with the dopaminergic system, hypothalamic-pituitary-adrenal (HPA) axis, and serotonergic system. Therefore, they hypothesized that MSCs could exert antidepressant and anxiolytic effects by modulating regions related to these systems. The results showed that, after MSC injection, the dorsal raphe nucleus (DRN) region of the serotonergic system exhibited significant neural activation (Fig. 1h–j) and a time-dependent increase in c-Fos expression (Supplementary Fig. 3g, h). As expected, MSC injection significantly increased the colocalization of c-Fos and 5-HT. In vivo electrophysiological recordings and fiber photometry in the DRN revealed that MSCs enhanced the firing frequency of 5-HT neurons and increased neuronal activity following CRS induction. Additionally, while CRS mice had lower brain levels of 5-HT, this level increased at least threefold after MSC injection (Fig. 1r and Supplementary Fig. 4h). Overall, these data indicate that intravenous MSC infusion activates 5-HT neurons in the DRN.
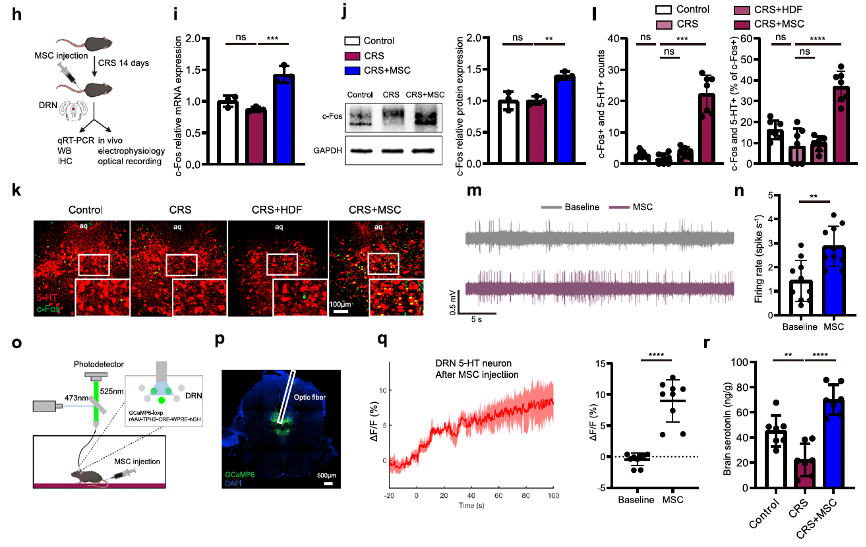
Fig. 1-2 Intravenous MSC infusion activates 5-HT neurons in the DRN.
Selective downregulation or inhibition of 5-HT neurons in the DRN nucleus does not cause significant anxiety-like behavior, but MSC injection fails to alleviate CRS-induced depressive-like behavior in different models of mice with DRN nucleus 5-HT neuron damage induced by 5,7-DHT or by virus-mediated expression of pro-apoptotic elements. This suggests that MSCs may exert their antidepressant and anxiolytic effects through 5-HTDRN neurons in the CRS model.
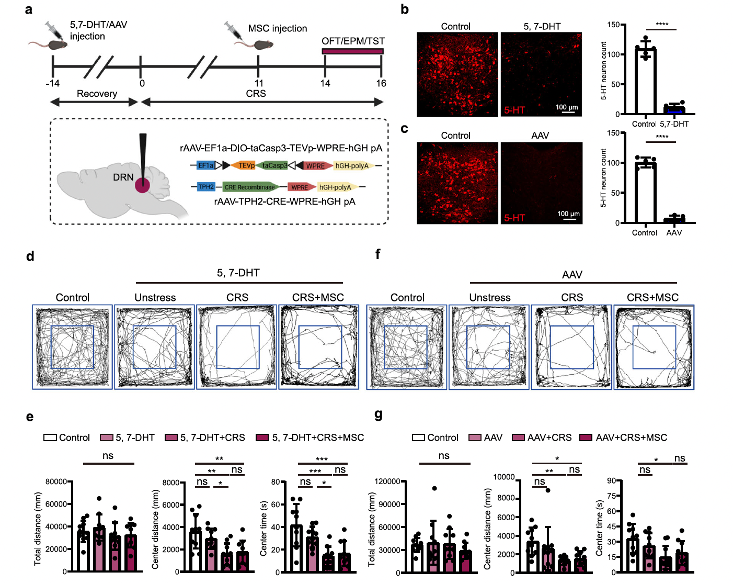
Fig. 2 MSCs potentially exert an antidepressant effect through 5-HTDRN neurons.
Is the activation of 5-HT neurons in the DRN caused by MSCs homing to the brain? Researchers intravenously injected GFP-labeled MSCs into stressed mice, and the results showed that MSCs primarily distributed in peripheral tissues, especially the lungs, and were not detected in the brain (Fig. 3a, b). The lungs are rich in various sensory nerve fiber terminals. Researchers used a modified lipid-permeable secreted fluorescent protein to label GFP-MSCs and detect their signals to directly identify their neighboring cells (Fig. 3c). After intravenous injection of labeled GFP-MSCs (GFP+mCherry+), sLP-mCherry was released and efficiently labeled the surrounding host tissue cells (Fig. 3d). They found that GFP-mCherry positive cells colocalized with the neuronal marker beta-III tubulin (Fig. 3e), suggesting that components secreted by MSCs might be taken up by pulmonary nerve fibers. The signals received in the lungs are primarily conducted by branches of the vagus nerve. Researchers hypothesized that MSCs might influence pulmonary vagal sensory neurons. As expected, they observed that MSCs could be located near VGLUT2+ (a marker for vagal sensory neurons) nerve fibers in the lungs (Fig. 3f). To further determine whether MSCs directly elicited responses in sensory fibers, they crossed VGLUT2-IRES-Cre mice with GCaMP6-loxp mice and recorded the activity of lung sensory fibers in ex vivo lung slices from VGLUT2-GcaMP6 mice (Supplementary Fig. 6a–c). They found that MSCs could stimulate vagal sensory fibers by measuring GCaMP6 fluorescence intensity (Fig. 3g and Supplementary Fig. 6d). To further confirm the positive response of vagal sensory neurons to MSC injection, they observed a significant increase in c-Fos expression in the nodose ganglion, where the cell bodies of vagal sensory neurons are located (Fig. 3h). Since vagal afferent fibers primarily project to the nucleus tractus solitarius (NTS) in the medulla, they detected the colocalization of c-Fos and 5-HT in NTS neurons (Fig. 3i, l) and performed vagotomy (left or right vagal nerves) before MSC injection (Fig. 3j). Their data showed that intravenous MSC administration activated neurons in the NTS, and unilateral vagotomy eliminated the activation of NTS neurons on the same side (Fig. 3i, k, l), ultimately reducing c-Fos signals and the colocalization of 5-HT and c-Fos in the DRN (Fig. 3m–o). In summary, these results indicate that MSCs in the lungs actively activate pulmonary afferent neurons, which may then convey information to the central nervous system (CNS).
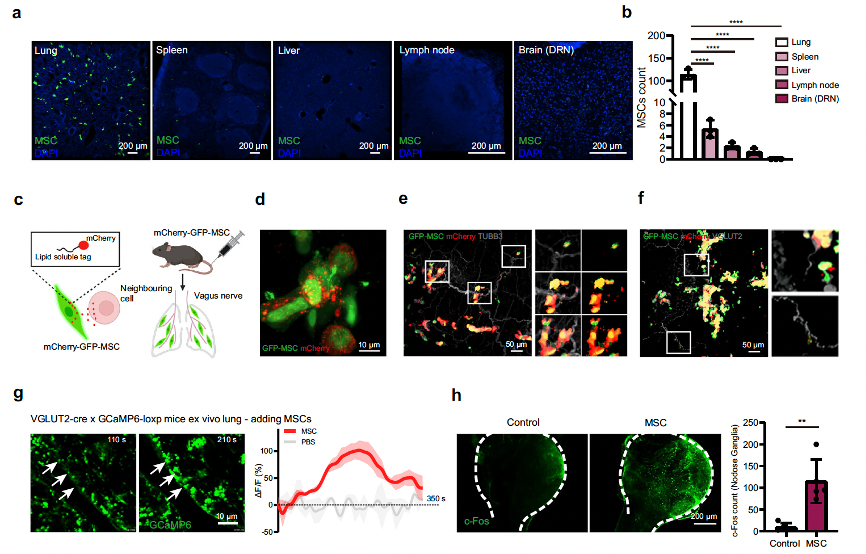
Fig. 3 Lung-innervating sensory neurons mediate the activation of 5-HTDRN neurons by peripheral MSCs. For more details, please refer to Figure 3 in the original text
The researchers used a recombinant pseudorabies virus (PRV) expressing enhanced green fluorescent protein (EGFP), which can retrogradely cross multiple synapses, to map the circuit from the DRN to the NTS and then to the lungs. The results showed that mice infected with PRV-EGFP exhibited bright GFP fluorescence in the fibers and cells of the DRN, and these cells were mainly 5-HT neurons (almost 80%). At 96 hours post-infection, the PRV had descended from the DRN to the medial vestibular nucleus, nucleus prepositus, NTS, nodose ganglia, and lungs. Additionally, overexpressing RFP-MSCs (RFP-MSCs) were located close to the PRV-EGFP-marked fibers in the lungs (Fig. 4g). To confirm this circuit, they injected an anterogradely transported, polysynaptic herpes simplex virus (HSV-EGFP) into the NTS (Fig. 4h, i), and found 5-HT-positive neuronal projection signals in the DRN (Fig. 4j). These two viruses used for multi-synaptic neuron labeling were provided by Brain Case Biotech, and before being released from the warehouse, they were tested in live animals at Brain Case's in vivo center.
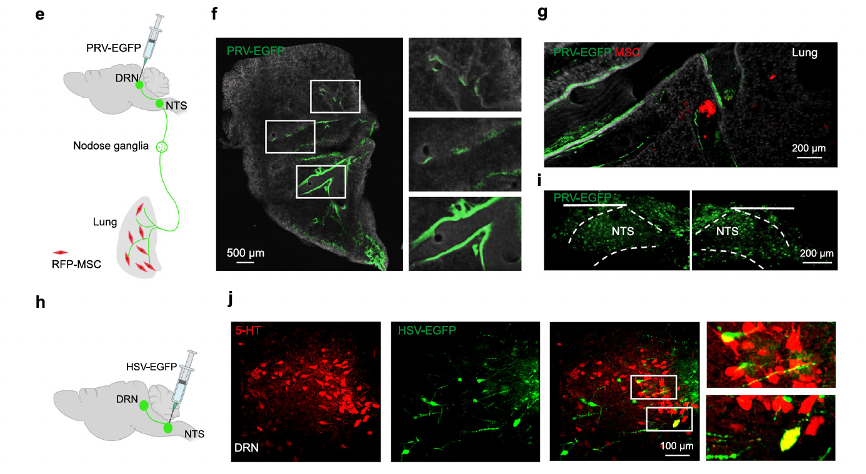
Fig. 4 MSCs relay neural signals to the DRN via pulmonary sensory nerves. For more experimental procedures and circuit tracing details, please refer to Figure 4 in the original text.
The researchers analyzed the transcriptional profiles of MSCs. Based on previous studies in authoritative databases, they screened and identified potential indicators involved in MSC-mediated activation of pulmonary afferent neurons. Among these indicators, the MSC-secreted protein brain-derived neurotrophic factor (BDNF) was found to be associated with major depressive and anxiety disorders. They detected specific and high-level expression of BDNF in GFP-positive MSCs at the protein level (details can be found in Supplementary Fig. 9a-c). More importantly, compared to the control group, MSCs significantly increased the phosphorylation levels of the BDNF receptor, TrkB, in VGLUT2 nerves (Fig. 5b, c). These findings suggest that peripheral MSCs may activate lung-innervating vagal sensory neurons through the BDNF-TrkB signaling pathway.
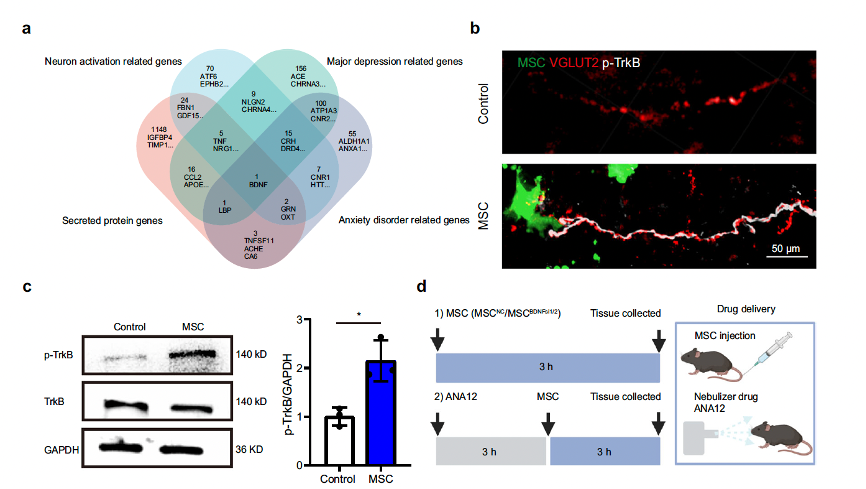
Fig. 5-1 MSCs activate 5-HT neurons via brain-derived neurotrophic factor(BDNF).
After knocking down the expression of BDNF in MSCs using RNA interference, the 5-HT neurons in the DRN could not be activated (Fig. 5h, i). When they investigated the importance of the BDNF-TrkB axis in MSC-mediated improvement of depressive and anxiety-like behaviors, they found that BDNF-knockdown MSCs could not restore the CRS-induced decreases in distance traveled and time spent in the center field of the open field test (OFT), time spent in and entry numbers to open arms in the elevated plus maze (EPM), and the increase in immobile time in the tail suspension test (TST) (Supplementary Fig. 10). To further confirm the results, they used ANA12, a small-molecule antagonist of TrkB. Administration of ANA12 by nebulizer inhalation before MSC infusion was found to abrogate MSC-mediated 5-HT neuronal activation (Fig. 5d, j, k). These data indicate that the BDNF-TrkB axis in the lungs is essential for MSC-mediated 5-HT neuronal activation, as well as their antidepressant and anxiolytic effects.
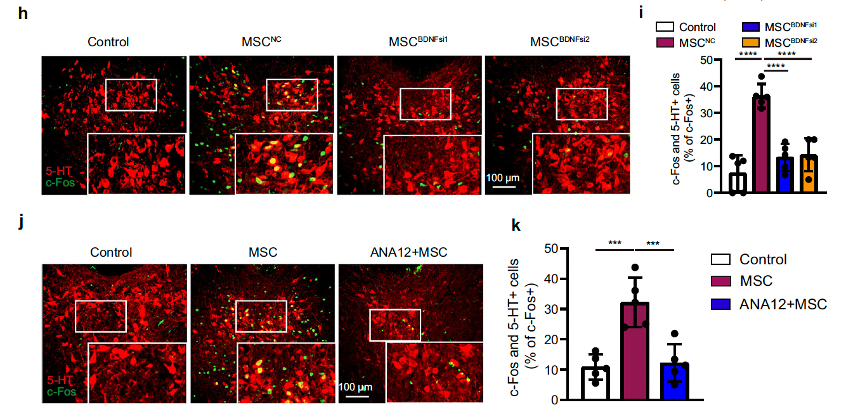
Fig. 5-2 MSCs activate 5-HT neurons via brain-derived neurotrophic factor(BDNF).
They hypothesized that inhalation of the TrkB agonist 7,8-DHF could improve depressive and anxiety-like behaviors. To explore this possibility, the researchers induced CRS depression model mice through 14 consecutive days of restraint stress and used a nebulizer to administer 10 mg of 7,8-DHF per kg of body weight, or an equal volume of vehicle, via inhalation (Fig. 6a). The data showed that 7,8-DHF inhalation treatment could activate 5-HT neurons in the DRN and improve brain 5-HT levels (Fig. 6b, c). Next, the therapeutic effects of 7,8-DHF inhalation were assessed on day 15 using the open field test (OFT), elevated plus maze (EPM), tail suspension test (TST), and forced swimming test (FST) (Fig. 6a). In the OFT, inhalation of 7,8-DHF significantly suppressed CRS-induced anxiety-like behaviors, as measured by increased distance traveled and time spent in the center field (Fig. 6d, f). Inhalation of the TrkB agonist also increased CRS mice’s preference for the open arms of the EPM, suggesting that 7,8-DHF inhalation treatment alleviated the anxiety-like behaviors caused by chronic restraint stress (Fig. 6e, g). Nebulized 7,8-DHF inhalation was also sufficient to rescue the depressive-like behaviors in this model, as indicated by the decreased immobility time in the TST and FST (Fig. 6h, i).
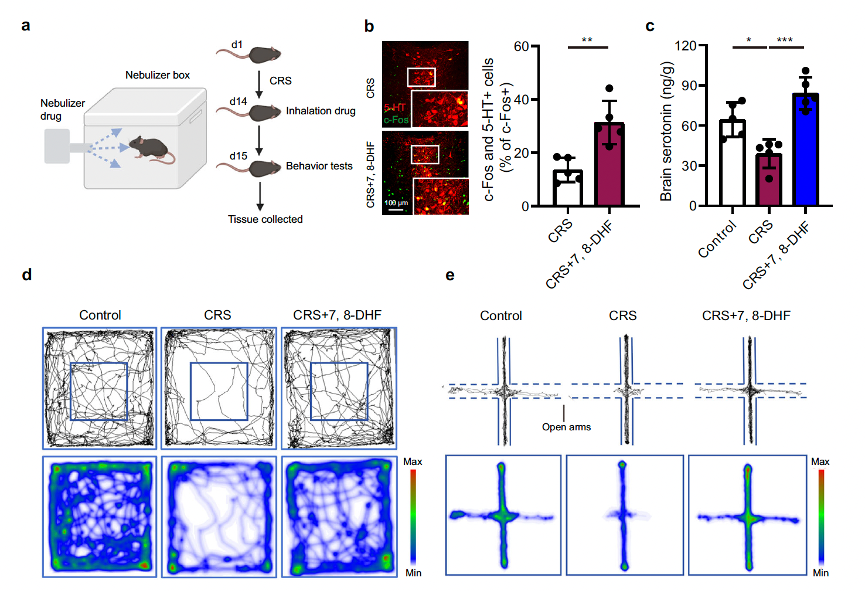
Fig. 6 Inhalation of a TrkB agonist as a promising depression and anxiety treatment in CRS mice.
Additionally, the researchers verified that 7,8-DHF primarily exerted its antidepressant and anxiolytic effects through the lung vagal-to-brain axis rather than through direct uptake via the olfactory epithelium/neurons into the brain.
For specific data, please refer to the original article: https://www.nature.com/articles/s41467-023-43150-0.