- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Optogenetics is an emerging technique for fundamental neurobiology. However, it is limited by its short tissue penetration and the requirement of invasive implantation of optical fibers. Here, a conversion nanotechnology is reported, where researchers achieve targeted motor neuron administration through a near-infrared (NIR) laser-triggered optogenetic approach to treat certain diseases caused by peripheral nerve injuries.
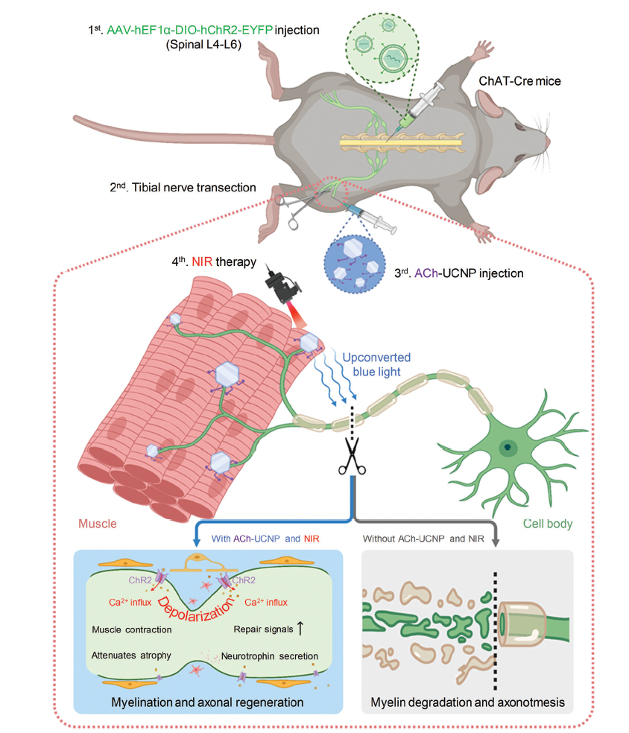
Fig 1. Schematic diagram of the tibial nerve completely-transected mouse model (the upper part) treated by the NIR-triggered upconversion optogenetic approach, with a zoomed-in view of a cascade of events following ChR2 activation (the lower part).
Previous studies have shown that nicotinic acetylcholine (ACh) receptors predominate on the postsynaptic membrane at the neuromuscular junctions of cholinergic motor neurons. Therefore, researchers synthesized ACh-modified UCNPs to target acetylcholine receptors on these specific neurons. Core-shell structured upconversion nanoparticles (UCNPs) were synthesized by co-doping Y3+, Yb3+, and Tm3+ using a thermal solvent method. The physical characteristics and upconversion fluorescence stability of the core-shell UCNPs, after surface modification with polyethylene glycol (PEG) and ACh, were analyzed using transmission electron microscopy (TEM) and high-angle annular dark field (HAADF) mode scanning transmission electron microscopy (STEM). The time-course targeting efficiency (including internalization) of the nanoparticles was monitored using inductively coupled plasma mass spectrometry (ICP-MS) after incubation in BV2 and skeletal muscle cell culture media. The in vitro cell biocompatibility of PEG-UCNPs and ACh-UCNPs was evaluated through standard cell viability assays. In vivo toxicity of PEG/ACh-UCNPs was assessed using hematology and blood chemistry tests. Overall, these results indicated that PEG-UCNPs and ACh-UCNPs possess relatively good biocompatibility, suggesting their potential biomedical applications in nerve injury models.
To determine whether ACh-UCNP was delivered to acetylcholine receptors on the postsynaptic membrane, researchers performed immunofluorescence histochemistry for colocalization confirmation. α-BTX was used to label acetylcholine receptors (AChR, pseudo-colored red), and UCNP fluorescence (pseudo-colored white) was detectable under 980 nm laser excitation. The results showed significant colocalization of fluorescence signals between ACh-modified UCNP and α-BTX (Figures 2D1, D2). In contrast, there was almost no fluorescence overlap between PEG-UCNP and α-BTX (Figures 2C1, C2). Researchers measured the tissue penetration depths of different wavelength lasers (including 475, 520, 660, and 980 nm) using an optical power meter. Due to the poor tissue penetration and high phototoxicity of the 475 nm laser, it was necessary to use UCNP as a light conversion medium between near-infrared light and visible light in vivo. Next, the power of upconverted blue light (wavelength at 475 nm) generated by UCNP in tissue penetration experiments was measured using a 980 nm laser (with the assistance of a 400–700 nm bandpass filter to avoid transmitting NIR, Figures S9D, E, Supporting Information). Combining all these parameters, they were able to estimate the practical power density of upconverted blue light irradiated on the tibial nerves in vivo (≈0.15 mW mm−2), which is sufficient to activate ChR2 for cation influx.
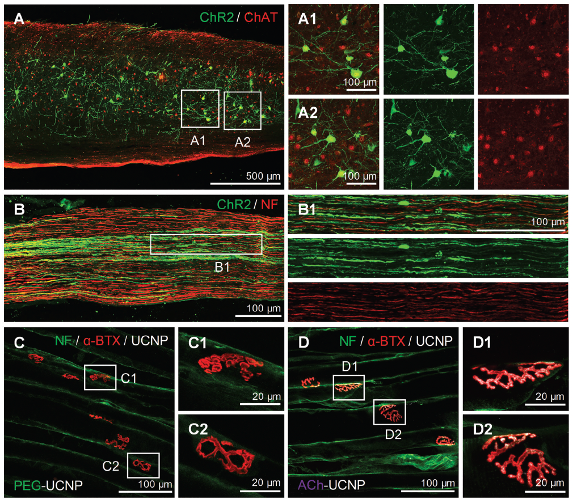
Fig 2. The expression of ChR2 in acetylcholinergic motor neurons and the targeting effect of ACh-UCNP on acetylcholine receptors
The anatomical images of tibial nerves (Figure 3) showed that only the transected nerves in the ACh-UCNP+NIR group were successfully reconnected after treatment. Clear disconnections were present in the tibial nerves of the other groups (injury without treatment, PEG-UCNP, ACh-UCNP, and PEG-UCNP+NIR), highlighted by red arrows. To further confirm the reconnection of the transected tibial nerves, retrograde nerve tracing was employed. The retrograde tracer, cholera toxin subunit B labeled with Alexa Fluor 488 (CTB-488), was injected into the gastrocnemius muscle after 6 weeks of therapy. For functional tibial nerves, the retrograde tracer CTB-488 could be reversely transported from the downstream tibial nerve terminal to the spinal cord, showing high co-localization with ChAT labeling of cholinergic motor neurons. These data demonstrated that the neural conduction of the completely transected tibial nerves in the ACh-UCNP+NIR group was reconstructed by our treatment method.
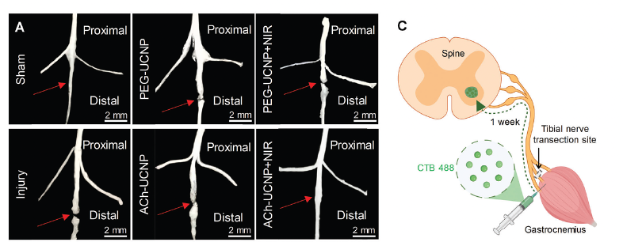
Fig 3. Regeneration of tibial nerve fibers promoted by UCNP-mediated optogenetics
Without effective recovery treatment aftermotor nerve injury and necessary exercise, the gastrocnemiusmuscle on the injured side would undergo atrophy leading to irreversible damages to the limb. According to the process shown in Figure 4A, the gastrocnemius muscles of the left (normal) and right (injured) posterior limbs of each group of mice were dissected at the end of treatment (6 weeks). They were photographed and weighed to evaluate muscle recovery after tibial nerve transection (Figure 4B,C). We calculated the wet weight ratios of the gastrocnemius muscles from the right posterior limbs to those from the left. The ratios were significantly reduced (≈0.7) in the injury without treatment, PEG-UCNP, ACh-UCNP, and PEG-UCNP+NIR groups, suggesting serious muscle atrophy due to inferior nerve recovery in these cases. In contrast, the ACh-UCNP+NIR group almost became identical to the Sham group in terms of the wet weight ratios of the gastrocnemius muscles (Figure 4C). The cross-sectional areas of myofibers and the distances between myofibers were further measured to evaluate the recovery of muscle atrophy between different groups. After 1, 3, and 6 weeks of treatment, the gastrocnemius muscles of each group were dissected for hematoxylin-eosin (HE) staining (Figure 4D). Statistical analysis of all the cross-sectional areas of myofibers in these images provided comprehensive profiles of gastrocnemius muscles during the treatment. Clearly, there was a trend of myofiber growth in the ACh-UCNP+NIR group, catching up with the Sham group after 6 weeks. In fact, the remaining groups featured moderately negative growth of gastrocnemius muscles under denervation (Figure 4E). These analyses validated that our approach of UCNP-mediated NIR optogenetics can most effectively promote the recovery of atrophic gastrocnemius muscles after tibial transection.
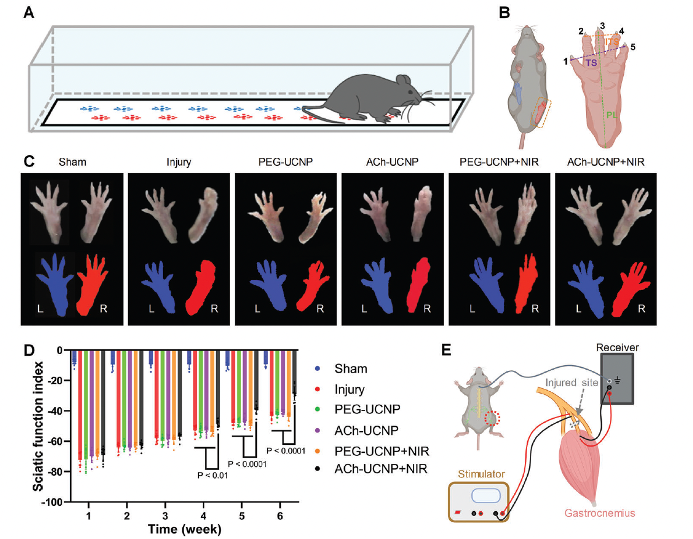
Fig 4. Improved motor function recovery and electrophysiological activities by UCNP-mediated optogenetics
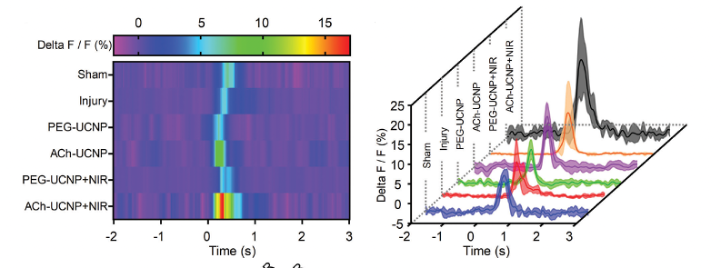
In this article, Brain Case Biotech's rAAV-hSyn-GCaMP6f virus was used to detect intracellular calcium influx in neurons, to assess the extent of repair in damaged neurons after NIR-Activated ChR2 treatment.