- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Under basal conditions, neurons primarily produce ATP through mitochondrial oxidative phosphorylation (OXPHOS). When neurons are activated and require higher energy demands, aerobic glycolysis predominates. This study revealed that PKM2 is a crucial molecule driving high levels of aerobic glycolysis in somata. In addition to leading to less oxygen consumption and more lactate production, the low pyruvate kinase activity of PKM2 redirects glucose metabolites into the pentose phosphate pathway, thereby maintaining antioxidant protection and nucleotide biosynthesis. Neurons can adapt their glucose metabolism to diverse metabolic needs at different stages and ages. Additionally, this study also found that neuronal somata exhibit higher levels of aerobic glycolysis and lower levels of OXPHOS under both basal and activated states. The glycolytic enzyme pyruvate kinase 2 (PKM2) is predominantly localized in the somata rather than in the terminals.
The researchers incubated isolated somata and synaptosomes with [1,2-13C]glucose and detected isotope-labeled glucose metabolic intermediates using mass spectrometry (Fig. 1a). The incorporation of [1,2-13C]glucose into glycolytic metabolites was higher in somata than in synaptosomes (Fig. 1b). They further cultured isolated somata and synaptosomes with 2-deoxy-d-glucose (2-DG) and found that the luminescence signal during somata lysis was stronger than in synaptosomes (Fig. 1c). These results indicate that glucose uptake and glycolytic flux are higher in somata than in nerve terminals. Further validation of metabolic differences between neuronal somata and synaptosomes was conducted using a Seahorse XF Analyzer. Consistent with the results of the metabolic flux experiment, the extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) showed that somata have higher glycolysis and lower basal respiration (Fig. 1f–i).
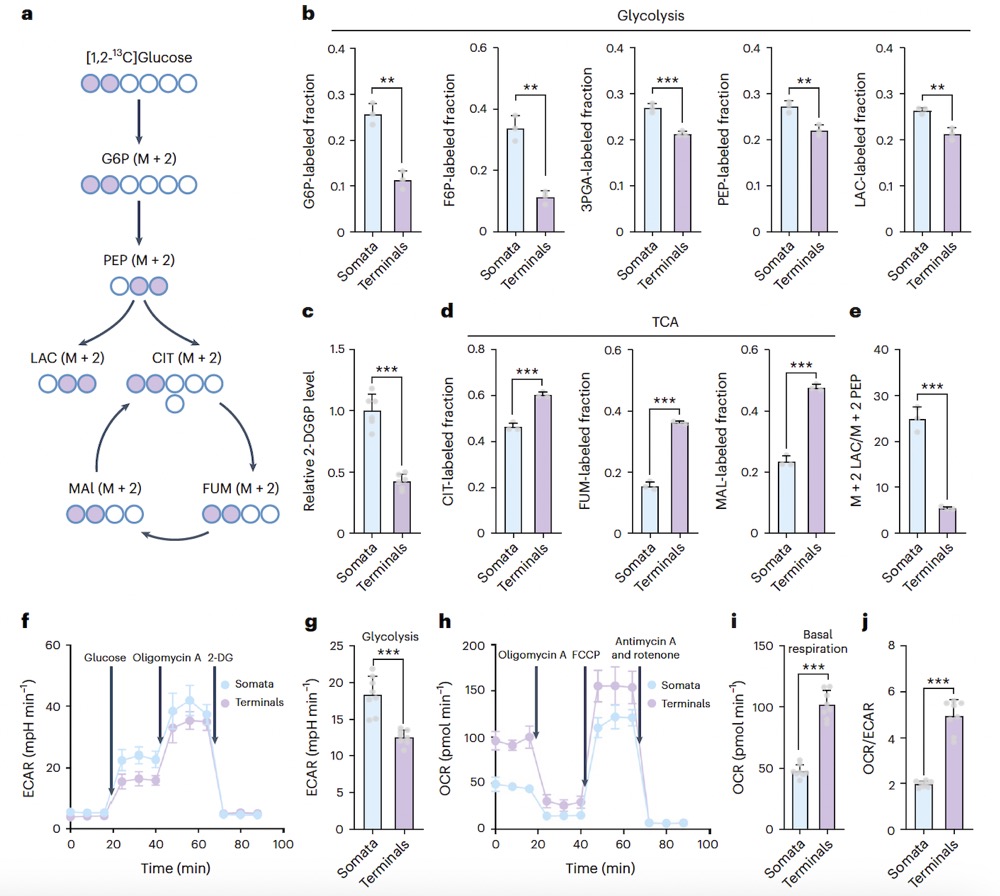
Fig1. Detection of isotope-labeled glucose metabolic intermediates using mass spectrometry (Fig. 1a); Cultivation of isolated somata and synaptosomes with 2-deoxy-D-glucose (2-DG) (Fig. 1c). Extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) in somata indicate higher glycolysis and lower basal respiration (Fig. 1f–i).
Using genetically encoded sensors combined with miniature two-photon microscopy, the researchers compared the contributions of OXPHOS and glycolysis to ATP levels in awake mice. They expressed cytosolic iATPSnFR (a sensor for detecting changes in intracellular ATP concentration, provided by Brain Case Biotech) in the primary somatosensory cortex (SSp) and dynamically monitored the effects of OXPHOS and glycolysis inhibitors to accurately measure their contributions to intracellular ATP production. They found that in somata, ATP levels significantly decreased only when glycolysis was inhibited. These results indicate that cortical neurons perform higher levels of aerobic glycolysis in somata than in nerve terminals. The researchers further explored the differences in glucose metabolism between somata and nerve terminals in resting and activated states. After injecting APV/CNQX to inhibit the spontaneous firing of cortical neurons, they found that somata perform more aerobic glycolysis, whereas nerve terminals perform more oxidative phosphorylation (OXPHOS) in the resting state.
Pyruvate is generated from phosphoenolpyruvate (PEP) catalyzed by pyruvate kinase31. There are two main isoforms of this enzyme in the brain, PKM1 and PKM2. We used Western blotting to detect levels of PKM2 in neurons from the cerebral cortex, substantia nigra, locus coeruleus, diagonal band nucleus, and cerebellum, finding that PKM2 levels were significantly higher in somata compared to synaptosomes, while PKM1 levels did not differ significantly between somata and synaptosomes. These results indicate that PKM2 levels are higher in somata than in terminals and are prevalent throughout the entire brain.
Downregulation of Pkm2 expression resulted in decreased levels of glucose-6-phosphate (G6P) and lactate, and increased levels of tricarboxylic acid (TCA) cycle intermediates such as citric acid, succinic acid, fumaric acid, and malic acid in the substantia nigra pars compacta (SNc). These findings indicate that deletion of Pkm2 in dopaminergic neurons shifts glucose metabolism in somata from aerobic glycolysis to oxidative phosphorylation. PKM2 emerges as a crucial molecule determining high levels of aerobic glycolysis in the primary somatosensory cortex (SSp) and SNc.
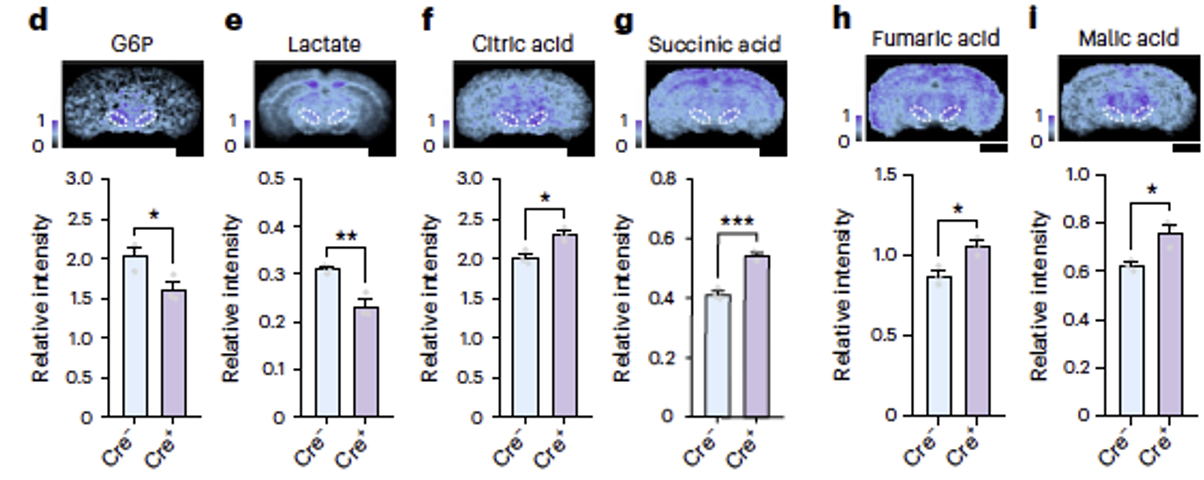
Fig2. Deletion of Pkm2 resulted in decreased levels of glucose-6-phosphate (G6P) and lactate in the substantia nigra pars compacta (SNc), and increased levels of citric acid, succinic acid, fumaric acid, and malic acid, which are intermediates of the citric acid cycle.
Following this, they investigated the reasons behind the high levels of aerobic glycolysis in somata. Compared to oxidative phosphorylation (OXPHOS), aerobic glycolysis does not generate reactive oxygen species (ROS), which helps prevent oxidative damage. Spatial metabolomics revealed a significant reduction in the antioxidants reduced glutathione and reduced ascorbic acid following Pkm2 gene deletion, indicating that changes in glucose metabolism in somata affected the neuronal redox buffering capacity. Therefore, we hypothesized that deletion of the Pkm2 gene leads to a shift in somatic glucose metabolism from aerobic glycolysis to oxidative phosphorylation, which resulted in increased lipid oxidative damage in cortical neurons and dopaminergic neurons. These findings suggest that somata use high levels of aerobic glycolysis to prevent oxidative damage.
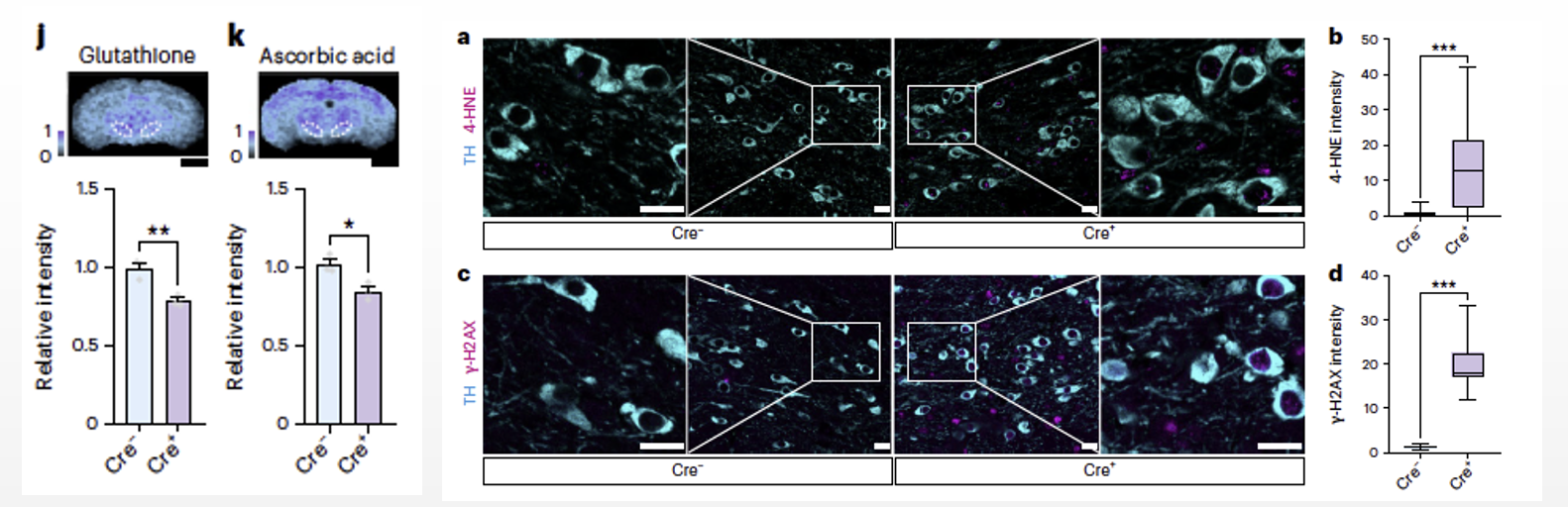
Fig6. After deletion of the Pkm2 gene, there was a significant reduction in the levels of the antioxidant reduced glutathione and reduced ascorbic acid (6j, k). In dopaminergic neurons, the shift in glucose metabolism patterns resulted in increased lipid and nucleic acid oxidative damage (6a–d).
For more details, please refer to the original article at https://www.nature.com/articles/s41593-023-01476-4
Catalog Number. BC-0622
Product Name. rAAV-hSyn-cyto-iATPSnFR1.0
Note: virus vector for detecting the release of intracellular ATP