- E-mail:BD@ebraincase.com
- Tel:+8618971215294
The mammalian cerebral cortex can be divided into six layers, which is an important structural basis for the brain to process sensory information. Compared with the superficial layers, the sixth layer of the cerebral cortex (layer 6, L6) is the earliest layer of structure that appears during development, but has been relatively rarely studied in depth. The cells of L6 are complex and diverse. There are two main types of pyramidal neurons in L6 of the sensory cortex of most species: corticothalamic (CT) neurons that project to the thalamus, and corticocortical (CC) neurons that project within the cortex. Although the specific markers and projection properties of L6 neurons have been partially revealed, how L6 neurons integrate inputs from different times and spaces on the dendrites to produce outputs remains unclear. This is mainly attributed to the high-quality function of L6 neurons. Technical difficulties in imaging.
On April 21, 2023, Zhang Jianxiong’s team from the Army Medical University jointly published a paper in the magazine "iScience" A research paper titled "Rabies virus-based labeling of layer 6 corticothalamic neurons for two-photon imaging in vivo" was published online. This study developed an RV-CVS virus-based labeling method that allows direct structural and functional imaging of projection-specific CT neurons in mouse auditory cortex L6 using conventional two-photon microscopy. This labeling method has the advantages of ultra-high brightness and ultra-fast expression within a week, and can be used for high-quality imaging of subcellular structures such as deep neuronal cell bodies and dendritic spines.

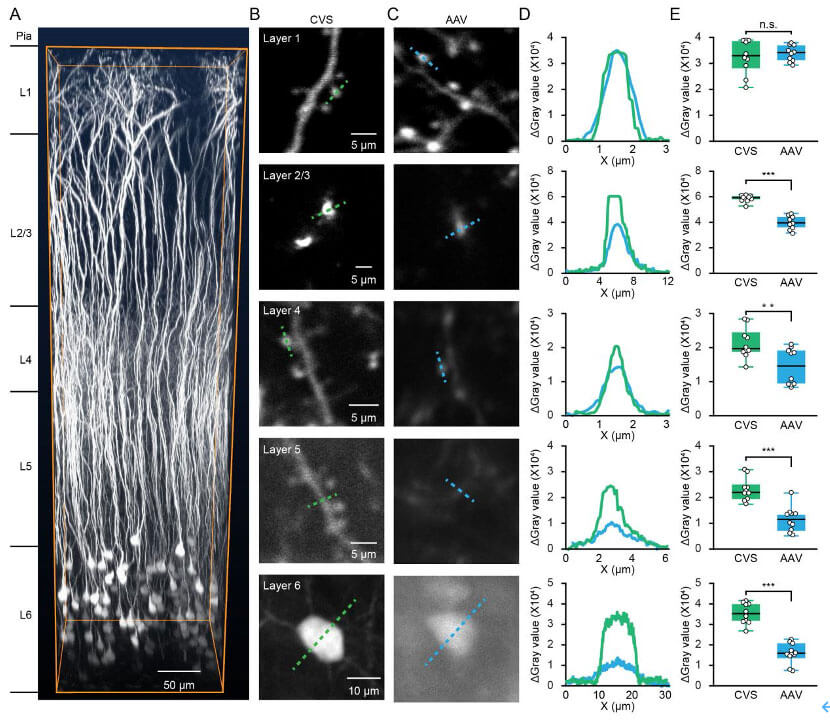
RV-CVS-labeled neurons have sufficiently high fluorescence intensity to enable high-contrast structural imaging of L6 neurons. The researchers used CVS-GFP to specifically label L6 neurons in the auditory cortex that project to the MGB. Compared with AAV-labeled neurons, the cell bodies, dendrites, and dendritic spines of RV-CVS-labeled neurons were more abundant in deeper layers. High fluorescence levels. Quantitative measurement of fluorescence intensity of multiscale structures in different layers showed that RV-CVS labeling was able to resolve individual dendritic spines of L5 and dendritic structures of L6. Therefore, RV-CVS labeling greatly improves the imaging quality of L6 CT neurons, enabling individual dendrites and dendritic spines to be studied in deeper layers.
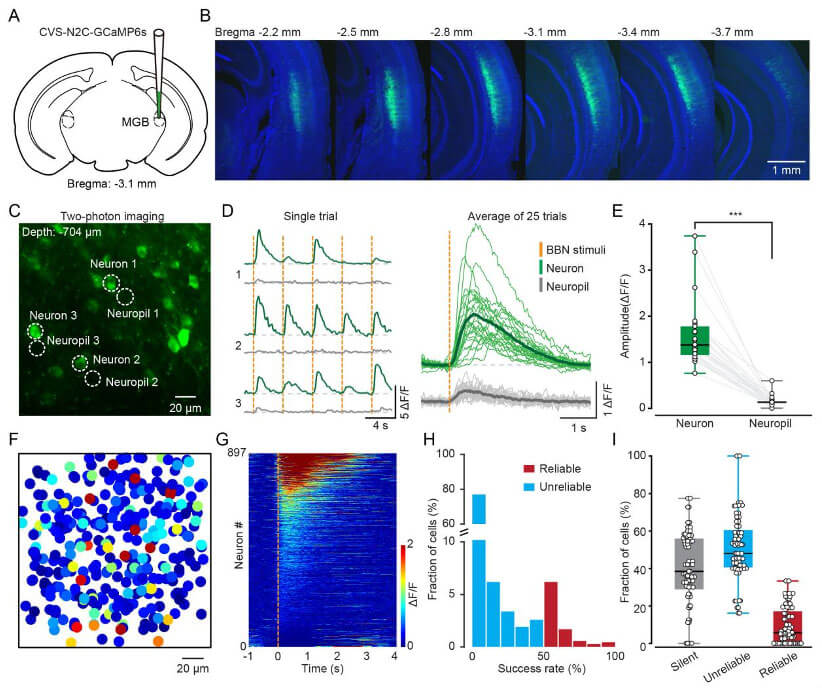
A common problem with two-photon in vivo population Ca2+ imaging is that neuronal signals are often interfered with by adjacent nerve fibers. To test whether RV-CVS labeling is subject to this interference, we used CVS-GCaMP6s to specifically label L6 neurons in the auditory cortex that project to the MGB and randomly sampled the signals from the neurons and their adjacent nerve fibers. The results showed that the response amplitude of neurons evoked by noise stimulation (BBN) was much higher than that of nearby nerve fibers. Next, the researchers performed functional Ca2+ imaging on neurons in the L6 CT population of awake mice, calculated the amplitude of each neuron's induction by BBN, and compared all 897 neurons based on their relative changes from pre-sound to post-sound. Sort by activity level. The above experiments show that CVS-GCaMP6s labeling can perform high-quality Ca2+ imaging of L6 CT neurons.
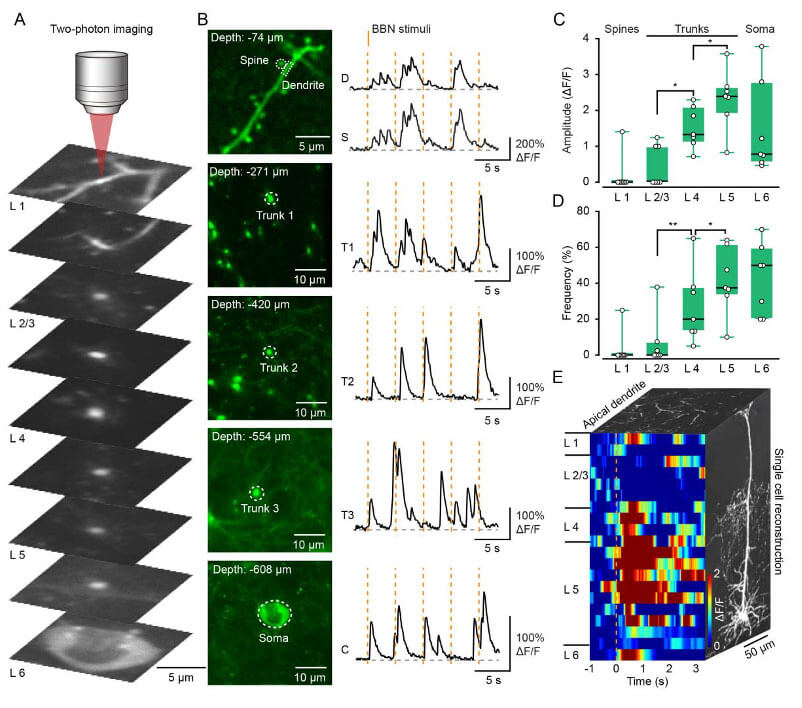
In addition to population imaging, RV-CVS labeling enables high-quality Ca2+ imaging of L6 single-cell structures spanning the entire cortex at subcellular resolution. The researchers recorded Ca2+ signals from different parts of the same neuron from superficial to deep layers and found that compared with the shallow layers, the amplitude and frequency of Ca2+ signals in the neuronal trunks located at L4 and L5 were greatly increased. The researchers recorded a total of 20 focal planes from shallow to deep on the same neuron, and completely reconstructed its functional map in the temporal and spatial dimensions to show the response characteristics of all structures. These suggest that RV-CVS labeling provides a suitable method to study dendritic information integration by imaging dendrites and somata of L6 CT neurons at single-cell resolution.
In summary, the researchers developed a neuron labeling strategy based on RV-CVS, which can perform high-contrast, multi-scale, high-quality structural and functional imaging of L6 CT neurons in the mouse auditory cortex.
Thanks to Dr. Gu for providing this document. Gu Miaoqing, a doctoral student at Guangxi University, is the first author of this paper. This work was strongly supported by the National Natural Science Foundation of China and the Scientific Instrument Development Project of the Chinese Academy of Sciences.
Original link: https://www.cell.com/iscience/fulltext/S2589-0042(23)00702-2#%20
All related products mentioned in the original article can be provided by Brain Case Biotech, as well as RV-CVS series products:
| Product name | Product number |
| RV-N2C(G)-ΔG-EGFP | BC-RV-N2C862 |
| CVS-N2c-ΔG-Gcamp6s | BC-RV-CVS715 |
| CVS-EnvA-ΔG-GCaMP6s | BC-RV-CVS EnvA715 |
| CVS-N2c-ΔG-tdTomato | BC-RV-CVS462 |